Mpbse Class 12 Physics Atomic Nucleus Solutions
Question 1. The Q -the value of a nuclear reaction A + b → C + d is defined by Q = [mA + mb-mC-md]c², where the masses refer to nuclear rest masses. Determine from the given data whether the following reactions are exothermic or endothermic
1. \({ }_1^1 \mathrm{H}+{ }_1^3 \mathrm{H} \rightarrow{ }_1^2 \mathrm{H}+{ }_1^2 \mathrm{H}\)
2. \({ }_6^{12} \mathrm{C}+{ }_6^{12} \mathrm{C} \rightarrow{ }_{10}^{20} \mathrm{Ne}+{ }_2^4 \mathrm{He}\)
Atomic masses are given to be
\(m\left({ }_1^1 \mathrm{H}\right)=1.007825 \mathrm{u}, m\left({ }_1^2 \mathrm{H}\right)=2.014102 \mathrm{u}\)
\(m\left({ }_1^3 \mathrm{H}\right)=3.016049 \mathrm{u}, m\left({ }_1^{12} \mathrm{C}\right)=12.000000 \mathrm{u}\)
\(m\left({ }_{10}^{20} \mathrm{Ne}\right)=19.992439 \mathrm{u}, \quad m\left({ }_2^4 \mathrm{He}\right)=4.002603 \mathrm{u}\)
Answer:
1. \({ }_1^1 \mathrm{H}+{ }_1^3 \mathrm{H} \rightarrow{ }_1^2 \mathrm{H}+{ }_1^2 \mathrm{H}\)
Q= \(m_{\mathrm{N}}\left({}_1^1\mathrm{H}\right)+m_{\mathrm{N}}\left({ }_1^3 \mathrm{H}\right)m_{\mathrm{N}}\left({ }_1^2 \mathrm{H}\right)-m_{\mathrm{N}}\left({ }_1^2 \mathrm{H}\right)\)
= \(m\left({ }_1^1 \mathrm{H}\right)-m_e+m\left({ }_1^3 \mathrm{H}\right)-m_e-m\left({ }_1^2 \mathrm{H}\right)\) + \(m_em\left({}_1^2\mathrm{H}\right)+m_e\)
= \(m\left({ }_1^1 \mathrm{H}\right)+m\left({ }_1^3 \mathrm{H}\right)-m\left({ }_1^2 \mathrm{H}\right)-m\left({ }_1^2 \mathrm{H}\right)\)
1.007825 + 3.016049-2 × 2.014102
= -0.00433 × 931.5 MeV = -4.03 MeV
∴ Q < 0, the reaction is endothermic
2. \({ }_6^{12} \mathrm{C}+{ }_6^{12} \mathrm{C} \rightarrow{ }_{10}^{20} \mathrm{Ne}+{ }_2^4 \mathrm{He}\)
Q = \(2 m_{\mathrm{N}}\left({ }_6^{12} \mathrm{C}\right)-m_{\mathrm{N}}\left({ }_{10}^{20} \mathrm{Ne}\right)-m_{\mathrm{N}}\left({ }_2^4 \mathrm{He}\right)\)
= \(=2 m\left({ }_6^{12} \mathrm{C}\right)-12 m_e-m\left({ }_{10}^{20} \mathrm{Ne}\right)+10 m_e\) \(-m\left({ }_2^4 \mathrm{He}\right)+2 m_e\)
= [2 × 12.000000-19.992439-4.002603] × 931.5 MeV
= 0.004958 × 931.5 MeV = 4.62 MeV
Q > 0; so, the reaction is exothermic.
Mpbse Class 12 Physics Atomic Nucleus Solutions
Question 2. Is the fission of 56Fe26 nucleus into two equal fragments, 28Al13 energetically possible? Argue by working out the Q of the process. Given, m ( 56Fe26 ) = 55.93494 u and m(28Al13) = 27.98191 u
Answer:
If possible, let the reaction be \({ }_{26}^{56} \mathrm{Fe} \rightarrow{ }_{13}^{28} \mathrm{Al}+{ }_{13}^{28} \mathrm{Al}\)
Q -value of the process = \(m\left({ }_{26}^{56} \mathrm{Fe}\right)-2 m\left({ }_{13}^{28} \mathrm{Al}\right)\)
55.934944-2 × 27.98191
= -0.02888 × 931.5 MeV
= -26.90 MeV
Since the Q -value is negative, fission is not possible
Question 3. The fission properties of 239Pu94 are very similar to those of 235U92 The average energy released per fission is 180 MeV. How much energy, in MeV, is released if all the atoms in 239Pu941 kg of pure undergo fission?
Answer:
Number of nuclei in 1 kg of 239Pu94
= \(\frac{6.023 \times 10^{23}}{239} \times 1000.0\)
= 2.52 × 10 24
The energy released per fission = 180 MeV
Total energy released = 2.52 x 1024 × 180 MeV
= 4.54 × 1026 MeV
Atomic Nucleus Class 12 Questions And Answers
Question 4. A 1000 MW fission reactor consumes half of its fuel in 5y. How much 235U92 did it contain initially? Assume that the reactor was active 80% of the time and all the energy generated arises from the fission of 235U92 and that this nuclide is consumed by the fission process.
Answer:
Energy generated per gram of 235U92
= \(\frac{6.023 \times 10^{23}}{235} \times 200 \times 1.6 \times 10^{-13} \mathrm{~J} \cdot \mathrm{g}^{-1}\)
Energy generated in 5 y
Power × Time × 80%
(1000 × 106) × (5 × 365 × 24 × 60 × 60) × 80% J
Amount of spent
= \(\frac{\left(1000 \times 10^6\right) \times(5 \times 365 \times 24 \times 60 \times 60)}{6.023 \times 10^{23} \times 200 \times 1.6 \times 10^{-13}} \mathrm{x} \times 235\)
= 1538 kg
Initial mass of 235U92 = 2 × 1538 kg = 3076 kg
Question 5. How long an electric lamp of 100 W can be kept glowing by fusion of 2.0 kg of deuterium? The fusion reaction can be taken as = \({ }_1^2 \mathrm{H}+{ }_1^2\mathrm{H}\rightarrow{}_2^3\mathrm{He}+\mathrm{n}+3.2 \mathrm{MeV}\)
Answer:
Number of nuclei in 2 kg of 2H1
= \(\frac{6.023 \times 10^{23} \times 2000}{2}=6.023 \times 10^{26}\)
Energy generated by the fusion of these nuclei
E = \(\frac{3.2 \times 6.023 \times 10^{26}}{2} \mathrm{MeV}\)
Power of the bulb = 100 W
Let the bulb = 100 W
Energy spent = \(\frac{100 \times t}{1.6 \times 10^{-13}} \mathrm{MeV}\)
∴ \(\frac{100 t}{1.6 \times 10^{-13}}=\frac{3.2 \times 6.023}{2} \times 10^{23}\)
t = \(\frac{3.2 \times 6.023 \times 10^{23} \times 1.6 \times 10^{-13}}{2 \times 100}\)
– 1.54 × 10 12 s
= 4.89 × 104 y
Nuclear Physics Class 12 Important Questions
Question 6. A source contains two phosphorus radionuclides 32P15 (T1/2 = 14.3 d) and 33P15 (T1/2 = 25.3 d). Initially, 10% of the decay comes from 33P15. How long one must wait until 90% do so. 33 32
Answer:
Let i R01 and R02 be the initial activities of 33P15 and 32P15 respectively and R1 and R2 be their activities at any instant t. According to the first observation
R01 = 10% (R01 +R02)
R02 = 9 R01 ……………………………. (1)
Again, R1 = 90% (R1 + R2)
Or, \(\frac{R_2}{R_{02}}=\frac{1}{81} \frac{R_1}{R_{01}}\) ……………………………. (1)
Combining equation (1) and (2)
⇒ \(\frac{R_2}{R_{02}}=\frac{1}{81} \frac{R_1}{R_{01}}\)
Or, \(\frac{R_{02} e^{-\lambda_2 t}}{R_{02}}=\frac{1}{81} \times \frac{R_{01} e^{-\lambda_1 t}}{R_{01}}\)
Or, \(81 e^{-\lambda_2 t}=e^{-\lambda_1 t}\)
⇒ \(\left(\lambda_2-\lambda_1\right) t=\dot{2} .303 \log 81\)
t = \(\frac{2.303 \log 81}{\frac{0.693}{14.3}-\frac{0.693}{25.3}}\)
Since Or, λ = 0.693T½
= 208 . 5 d
Question 7. Under certain circumstances, a nucleus can decay by = 231.1 MeV emitting a particle more massive than an a -particle. Consider the following decay processes
1. \({ }_{88}^{223} \mathrm{Ra} \rightarrow{ }_{82}^{209} \mathrm{~Pb}+{ }_6^{14} \mathrm{C}\)
2. \({ }_{88}^{223} \mathrm{Ra} \rightarrow{ }_{86}^{219} \mathrm{Rn}+{ }_2^4 \mathrm{He}\)
Calculate the Q -values for these two decays and determine that both are energetically possible.
m( 223Ra88 ) = 223.01850 u, m( 209Ra82 ) = 208.98107 u,
m(219Ra86 ) = 219.00948 u, m(14C6) = 14.00324 u and m(4He2) = 4.00260 u
Answer:
1. \({ }_{88}^{223} \mathrm{Ra} \rightarrow{ }_{82}^{209} \mathrm{~Pb}+{ }_6^{14} \mathrm{C}+Q\)
= \(\left[m_{\mathrm{N}}\left({ }_{88}^{223} \mathrm{Ra}\right)-m_{\mathrm{N}}\left({ }_{82}^{209} \mathrm{~Pb}\right)-m_{\mathrm{N}}\left({ }_6^{14} \mathrm{C}\right)\right]\) x 931.2 MeV
= \(\left[m_{88}^{223} \mathrm{Ra}-m\left({ }_{82}^{209} \mathrm{~Pb}\right)-m\left({ }_6^{14} \mathrm{C}\right)\right] \times 931.2 \mathrm{MeV}\)
31. 8 MeV
∴ Q > 0: so, the decay is possible
2. \({ }_{88}^{223} \mathrm{Ra} \rightarrow{ }_{86}^{219} \mathrm{Rn}+{ }_2^4 \mathrm{He}+Q^{\prime}\)
Q’ = 5.98 MeV [by similar calculation as above]
∴ Q’ > 0; so, this decay is also possible.
Nuclear Physics Class 12 Important Questions
Question 8. Consider the fission of 238U92 by fast neutrons. In one fission event, no neutrons are emitted and the final stable end products, after the beta decay of the primary fragments, are140Ce58 and 99Ru44 Calculate Q for this fission process
Given
\(m\left({ }_{92}^{238} \mathrm{U}\right)=238.05079 \mathrm{u}, m\left({ }_{58}^{140} \mathrm{Ce}\right)=139.90543 \mathrm{u}\)
\(m\left({ }_{44}^{99} \dot{\mathrm{Ru}}\right)=98.90594 \mathrm{u}, \dot{m}_n=1.008667 \mathrm{u}\)
Answer:
The fission reaction is
⇒ \({ }_{92}^{238} \mathrm{U}+{ }_0^1 \mathrm{n}{\beta}{ }_{58}^{140} \mathrm{Ce}+{ }_{44}^{99}\mathrm{Ru}+Q\)
∴ Q = \(\left[m\left({}_{92}^{238}\mathrm{U}\right)+m\left({ }_0^1\mathrm{n}\right)m\left({}_{58}^{140}\mathrm{Ce}\right)-m\left({ }_{44}^{99} \mathrm{Ru}\right)\right] \mathrm{u}\)
= \([238.05079+1.00867-139.90543-98.90594]\)
= 23 1.1 MeV
Question 9. Suppose India had a target of producing by 2020 AD, 200, 000 MW of electric power, 10% of which was to be obtained from nuclear power plants. Suppose we are given that, on average, the efficiency of utilization (i.e., conversion to electric energy) of thermal energy produced in a reactor was 25%. How much amount of fissionable uranium did our country need per year by 2020? Take the heat per fission of 235U92 to be about 200 MeV. Avogadro’s number = 6.023 × 10 mol-1. Nuclear power target = 10% of total generation
Answer:
Nuclear power target = 10% of total generation
= 10% × 2 × 1011 W
= 2 × 1010 W
Efficiency, η = 25%
∴ Total power generated by the nuclear reactor
= \(\frac{2 \times 10^{10}}{25 \%} \mathrm{~W}=8 \times 10^{10} \mathrm{~W}\)
∴ Generated heat by the reactor in 2020
H = 8 × 1010 × 366 × 24 × 60 ×60 J
∴ Number of fission required for generation of this heat,
N = \(\frac{H}{200 \times 1.6 \times 10^{-13}}\)
If m g of 235U92 contains this number of nuclei, then,
m = \(\frac{N \times 235}{6.023 \times 10^{23}} \mathrm{~g}\)
= \(\frac{8 \times 10^{10} \times 366 \times 24 \times 60 \times 60 \times 235}{200 \times 1.6 \times 10^{-13} \times 6.023 \times 10^{23}} \mathrm{~g}\)
= 3.084 × 104 kg
Structure Of Atomic Nucleus Class 12 Questions
Question 10. Calculate and compare the energy released by
- Fusion of: 1.0 kg of hydrogen deep within the sun and
- The fission oqc of 1.0 kg of 235U in a fission reactor.
Answer:
1. Equation of fusion reaction,
4 1H → 4He2 + 2 0e+1 + 26 MeV
∴ 26 MeV of energy is released on a combination of four H nuclei.
Number of nuclei in 1 kg of hydrogen
= 6.023 × 1023 × 1000
Energy released,
EH = 6.023 × 1026 × 26 MeV
2. Fission of one nucleus releases 200 MeV of energy
Number of nuclei in 1 kg of 235U92 = \(\frac{6.023 \times 10^{23} \times 1000}{235}\)
∴ Energy released,
EU = \(\frac{6.023 \times 10^{23} \times 1000 \times 20}{235}\)
= 5.12 × 1026 MeV
∴ \(\frac{E_{\mathrm{H}}}{E_{\mathrm{U}}}=\frac{3.913 \times 10^{27}}{5.12 \times 10^{26}}\)
= 7.6
Question 11. The half-life of a radioactive substance is 30 days. The number of atoms in the substance is 1012. How many disintegrations of atoms per second does occur?
Answer:
T = 30 d =30 × 24 × 60 × 60 s
⇒ \(\lambda=\frac{0.693}{T}\)
= \(=\frac{0.693}{30 \times 24 \times 60 \times 60}\)
= 2.67 × 10-7(approx)
If t = 1 s, t = 2.67 × 10-7
Hence, eλt = 1.000000267(approx)
N = N0eλt = \(\)
= 9.99999733 × 1011 (approx)
∴ Number of atoms disintegrated per second
= N0 – N = 2.67 × 10 5 (approx)
Question 12. Draw a plot of the potential energy of a pair of nucleons as a function of their separations. Mark the region where the nuclear force is (1) attractive and (il) repulsive. Write any two characteristic features of nuclear forces
Answer:
The required plot
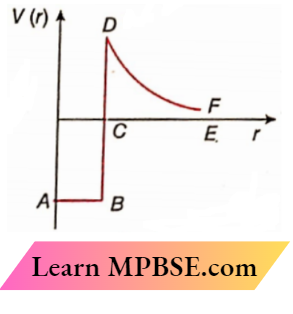
In region AB, nuclear force is attractive.
The nuclear force is not repulsive. The repulsive force corresponding to the region DF is the repulsive coulomb force between protons
Structure Of Atomic Nucleus Class 12 Questions
Question 13.
- Draw the Plot of binding energy per nucleon (B.E./A ) as a function of mass number A . Write two important conclusions that can be drawn regarding the nature of
nuclear force
- Use this graph to explain the release of energy in both the processes of nuclear fusion and fission.
- Write the basic nuclear process of neutrons undergoing β-decay. Why is the detection of neutrinos found very difficult?
Answer:
1.
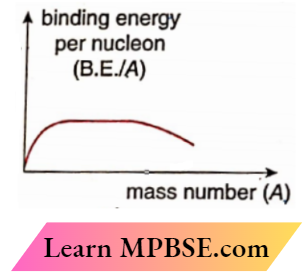
- Two conclusions from the plot: the nuclear force is
- Short-range and
- Charge independent
2. Mass of a nucleus (M) = mass of its nucleons- binding energy (B)
So, M decreases with an increase in B.
Now, we consider nuclear fission: 1 → 2 + 3.
From mass-energy equivalence,
M1 = M2 + M3+ energy release (Q)
or, Q = M1 – M2 – M3
Q is positive, i.e., energy is released if
M1 > M2 – M3,i.e., B1 < B2 – BM3
or A1e1<A2e2 + A3e3
Where A1A2, A3 are mass numbers A1 = A2 + A3) and e1 , e2 , e3 are binding energy per nucleon. From the plot, we see that this condition is satisfied for high A1, where both e2 and e3 are higher than e1 of the large nucleus 1.
The fission of a large nucleus releases energy. On the other hand, for low A nuclei, e2, and e3 will be less than the e1 of the larger nucleus 1. So, energy will be released rather in the opposite process: 2 + 3 → 1. Therefore, a fusion of small nuclei releases energy
3. It is very difficult to detect neutrinos or antineutrinos experimentally because they have neither any charge nor any mass.
Question 14. Define the activity of a radioactive sample. Write its SI unit. A radioactive sample has the activity of 10000 disintegrations per second (DPS) after 20 hours. After the next 10 hours its activity reduces to 5000 dps. Find out its half-life and initial
The activity of a radioactive sample is defined as the rate of disintegration of the sample. It is also called the count rate. Its SI unit is becquerel (Bq).
1 Bq = 1 decays/s
We know A = A8 e-λt
5000 = 10000e-λt
Or, eλt = \(\frac{10000}{5000}\)
= 2
Taking logs on both sides we get
λt = log 2
Or, λ = \(\frac{\log 2}{t}\)
Now half-life T = \(=\frac{0.693}{\lambda}=\frac{0.693 \times t}{\log 2}=\frac{0.693 \times 600}{\log 2}\)
= 1381. 26 min 23 h
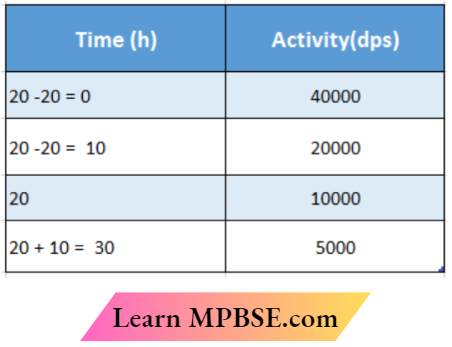
In this table, the last two values are the given values. Prom these values, the first two values have been calculated,
Atomic Nucleus Class 12 Questions And Answers
Question 15.
1. A radioactive nucleus ‘A ‘ undergoes a series of decays as given below:

The mass number and atomic number of A2 are 176 and 71 respectively. Determine the mass and atomic numbers of A4 and A.
2. Write the basic nuclear processes underlying α and β decays
Answer:
1. When β– decay occurs

When β+ decay occurs

In the case of β– decay, the mass number of A is 180 and its atomic number is 72. In the case of β+ decay, the mass number of A is 100 and its atomic number is 74. In both cases, the mass number of A4 Is 172 and its atomic number is 69.

Question 16. Explain the processes of nuclear fission and nuclear fusion by using the plot of binding energy per nucleon (BE/A) versus the mass number A
Nuclear fission:
The binding energy per nucleon for heavier nuclei is approximately 7.6 MeV, but for lighter nuclei, it is roughly 8.4 MeV. The heavier nuclei exhibit reduced stability, leading to the fission of the heavier nucleus into lighter nuclei, which therefore releases energy. This process is referred to as nuclear fission.
Nuclear fusion:
The binding energy per nucleon for nuclei with mass number A < 12 is minimal, rendering them less stable. Consequently, two such nuclei can amalgamate to create a somewhat heavier nucleus, which possesses a greater binding energy per nucleon.
Consequently, energy is liberated in this process, referred to as nuclear fusion.
Atomic Nucleus Class 12 Questions And Answers
Question 17. A radioactive isotope has a half-life of 10 years. How long will it take for the activity to reduce to 3.125%?
Answer:
Activity A = λN
Given A = 3. 125 % of \(\frac{3.125 A_0}{100}=\frac{A_0}{32}\)
Or, \(\frac{A}{A_0}=\left(\frac{1}{2}\right)^5\)
∴ \(\frac{N}{N_0}=\left(\frac{1}{2}\right)^5\)
So the activity will reduce to 3.125% after 5 half-lives. Hence required time =5 × 10 = 50 years
