- Chapter 1 Knowing Our Numbers
- Chapter 2 Whole Numbers
- Chapter 3 Playing With Numbers
- Chapter 4 Basic Geometrical Ideas
- Chapter 5 Understanding Elementary Shapes
Question 1. What are plant hormones?
Answer:
Plant hormones or phytohormones are non-nutrient diffusible chemical substances that control the activities of plants like growth, development, differentiation, movements, and other physiological processes.
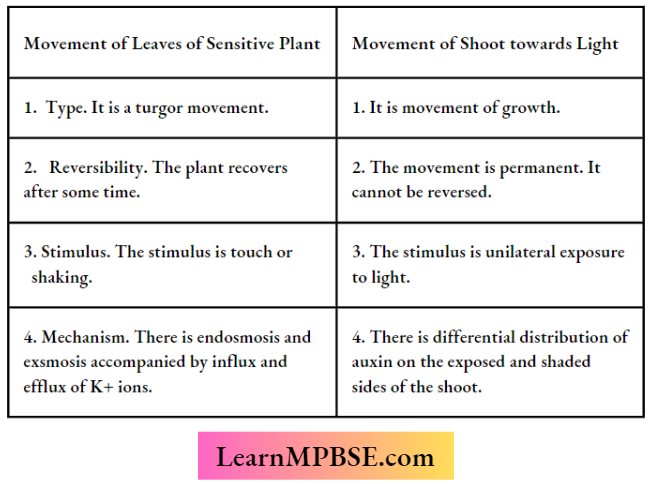
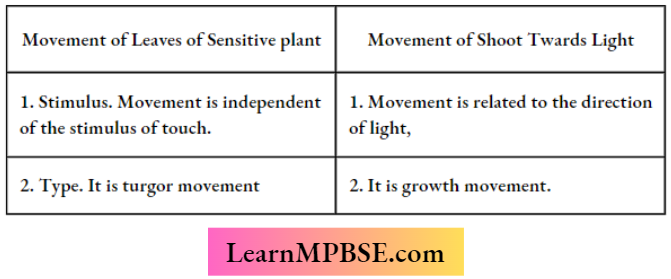
Question 2. How is the movement of the leaves of a Sensitive Plant different from the movement of the shoot toward light?
Answer:
Question 3. Give an example of a plant hormone that promotes growth.
Answer: Auxin (IAA)/Gibberellins (GA).
Question 4. How do auxins promote the growth of a tendril around a support?
Answer:
MPBSE Class 10 Science Chapter 7 Coordination In Plants Question and Answers
Question 5. Explain the cause of shoots of the plant bending towards light.
Answer:
Question 6. What are nastic and curvature movements? Give one example of each.
Answer:
Question 7.
Answer:
Question 8.
Answer:
Question 9. How is the movement of leaves of sensitive plants different from the movement of a shoot towards light?
Answer:
MPBSE Class 10 Science Chapter 7 Coordination In Plants Question and Answers
Question 10. Which plant hormone
Answer:
Question 11. What is phototropism?
Answer:
Phototropism. It is the directional growth movement of curvature that occurs in plant organs in response to unilateral light.
Question 12. What is hydrotropism?
Answer:
Hydrotropism. It is the tropic or directional movement of curvature that occurs due to unilateral exposure to water. Roots are positively hydrotropic.
Question 13. If you keep the potted plant horizontally for 2-3 days, what type of movements would be shown by the shoot and root after 2-3 days? Why?
Answer:
Question 14.
Answer:
MPBSE Class 10 Science Chapter 7 Coordination In Plants Question and Answers
Question 15. Why does the shoot of the plant bend towards light when it is kept inside a cardboard box with a small hole?
Answer:
Question 16. What is geotropism? Describe an experiment to demonstrate positive and negative geotropism.
Answer:
Geotropism. It is a tropic or growth movement of curvature which occurs in response to the vector of gravity. The main stem is generally negatively geotropic while the main root is positively geotropic.
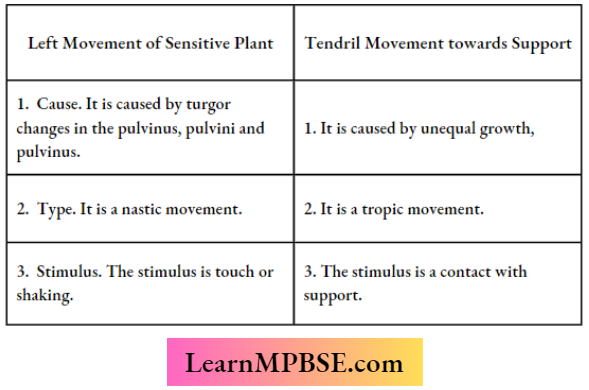
Question 17. List in tabular form three differences in the movement of leaves of Sensitive Plants when touched and the movement of tendrils towards the support.
Answer:
Question 18.
Answer:
Question 19. How does the sensitive plant detect the touch and how do the leaves move in response?
Answer:
Question 20. What are plant hormones? Name the plant hormones responsible for the following :
Answer: Plant hormones are non-nutrient, diffusible, chemical substances that control and coordinate growth, movements, and development.
MPBSE Class 10 Science Chapter 7 Coordination In Plants Question and Answers
Question 21.
Answer:
Touching the sensitive plant creates an electrochemical impulse that travels from cell to cell quickly and reaches the bases of leaflets and leaves.
Special cells present at these bases shrink and cause bending movement of leaves and leaflets. Recovery occurs in about ten minutes when basal cells regain turgidity.
Question 1. In a monohybrid cross between tall Pea plants TT and short Pea plants tt, a scientist obtained only tall Pea plants Tt in the F1 generation. However, on selling the F1 generation Pea plants he obtained both tall and short plants in the F2 generation. Based on the above observations and with other angiosperms also, can the scientist arrive at a law? If yes, explain the law. If not, give a justification for your answer.
Answer:
There are two possibilities for the occurrence of tall plants in the F1 generation :
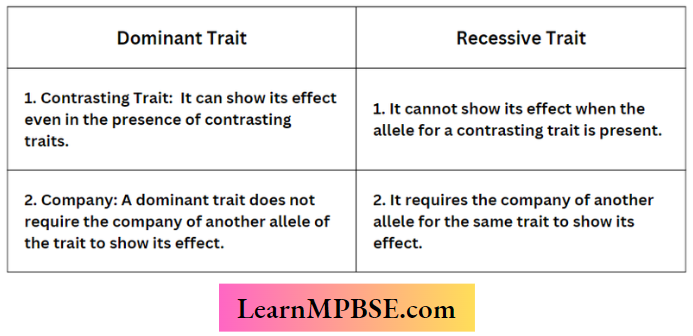
Question 2.
Differences Between Dominant And Recessive Traits
Answer: (1)
2. 75% of plants had round seeds while 25% of plants had wrinkled seeds. The ratio is 3: 1.
MPBSE Class 10 Chapter 9 Heredity Question And Answers
Question 3. “A trait may be inherited but may not be expressed.” Justify the statement with the help of a suitable example.
Answer:
Question 4. Name the plant Mendel used for his experiment. What type of progeny was obtained by Mendel in F1 and F1 generations when he crossed tall and short plants? Write the ratios he obtained in F2 generation plants.
Answer:
Garden Pea (Pisum sativum)
F1 Generation. All tall.
F2 Generation. 3 tall: 1 short or 3: 1
or 1 pure tall: 2 hybrid tall: 1 dwarf or 1: 2: 1.
Question 5.
Answer:
Class 10 Chapter 9 Heredity Question And Answers
Question 6. A green-stemmed rose plant denoted by GG and a brown-stemmed rose plant denoted by GG are allowed to undergo a cross with each other.
1. List your observations regarding (z) the Colour of the stem in the F1 progeny and the Percentage of brown stemmed plants in F2 progeny if F1 plants are self-pollinated. The ratio of GG and Gg in F2 progeny.
2. Based on the findings of this cross, what conclusion can be drawn?
Answer:
Conclusion: The trait which is expressed in whole F1 progeny is dominant while the other trait which remains unexpressed in F1 progeny but reappears in F2 progeny is recessive.
Question 7.
Answer:
Question 8. Mendel crossed two plants with visible contrasting characteristics and found that there were no halfway characteristics in the plants of F1 progeny. Explain the reason for this observation of Mendel.
Answer:
Despite having inherited both the contrasting traits, F1 progeny shows the trait of only one parent. This is because in the hybrid (F1 plants) only one trait expresses its effect. The trait is dominant. The other trait which does not express its effect in the hybrid is called recessive.
Class 10 Chapter 9 Heredity Question And Answers
Question 9. What are chromosomes? Explain stability of the DNA of the species is ensured in sexually reproducing organisms.
Answer:
Question 10.
Answer:
Question 1. (1) How many times does the blood go through the heart during one cycle in fish and why?
{2) List the respiratory pigment present in our body. Where is it present?
(3) Why are valves present in the heart and veins?
Answer:
Question 2. (1) Explain in brief the mechanism of circulation of blood in the human body.
(2) “Lymph is another type of fluid involved in transportation.” Justify the statement by explaining the process.
Answer:
(1) The right atrium receives deoxygenated blood from the heart (coronary sinus), upper part of the body (superior vena cava), and middle and lower part of the body (inferior vena cava). The left atrium receives oxygenated blood from the lungs.
(2) Lymph is a transportation fluid formed from tissue fluid. It is specialised to collect large size secretions and excretions which cannot directly pass into the blood, For Example., proteins, hormones, and fat. The lymph picks them and pours their contents into the blood in the region of the subclavian vein.
MPBSE Class 10 Science Life Processes Question and Answers
Question 3. (1)(A) Receives deoxygenated blood from vena cava (B) Sends deoxygenated blood to the lung through the pulmonary artery (C) Receives oxygenated blood from lungs and (D) Sends oxygenated blood to all parts of the body through the aorta.
(2) What does the blood consist of?
(3) Name the respiratory pigment in human beings and discuss its role.
Answer:
Question 4. What is lymph? How is the composition of lymph different from blood plasma? What is the direction of its flow? List two functions of the lymphatic system.
Answer:
Lymph is a straw-coloured viscous fluid which is formed from tissue fluid and flows inside tubes called lymph vessels.
Lymph differs from blood plasma in having :
Direction of Flow. Unidirectional from tissues all over the body to subclavian veins.
Functions of Lymphatic System.
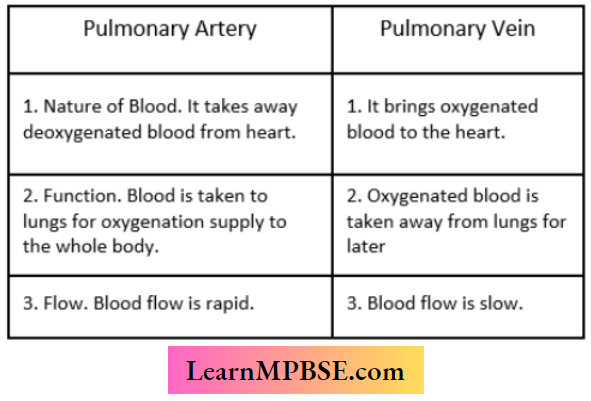
Question 5. (A) What do the following transport: (1) Xylem (2) Phloem (3) Pulmonary vein (4) Vena Cava?
(B) Write two points of difference between the pulmonary artery and the pulmonary vein.
Answer:
MPBSE Class 10 Science Life Processes Question and Answers
Question 6. (1) The upward movement of water normally requires a pump in our houses but in tall trees, water rises up without any external support. Explain the mechanism.
(2) State three points of difference between the transport of materials in the xylem and phloem.
Answer:
(1) Water rises up the top of the tallest plants through the development of a negative pressure caused by loss of water in transpiration. Transpiration or loss of water in vapour form occurs from mesophyll and other cells of aerial parts.
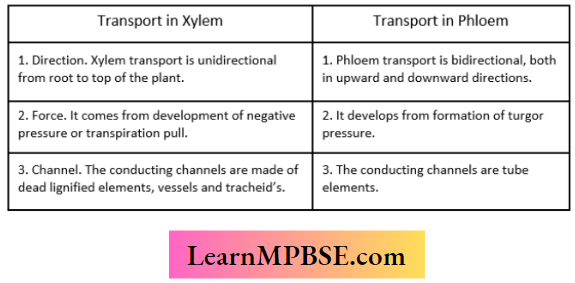
2. Force. It comes from the development of negative press- 2. It develops from the formation of turgor pressure, sure or transpiration pull.
3. Channel. The conducting channels are made of the conducting channels are formed of living sieves dead lignified elements, vessels and tracheids. tube elements.
Question 7. (1) Mention any two components of blood.
(2) Trace the movement of oxygenated blood in the body.
(3) Write the function of valves present in between atria and ventricles.
(4) Write one structural difference between artery and vein.
Answer:
MPBSE Class 10 Science Life Processes Question and Answers
Question 8. (1) Write two water-conducting elements present in plants. How does water enter continuously into the root system?
(2) Explain why plants have low energy needs as compared to animals.
Answer:
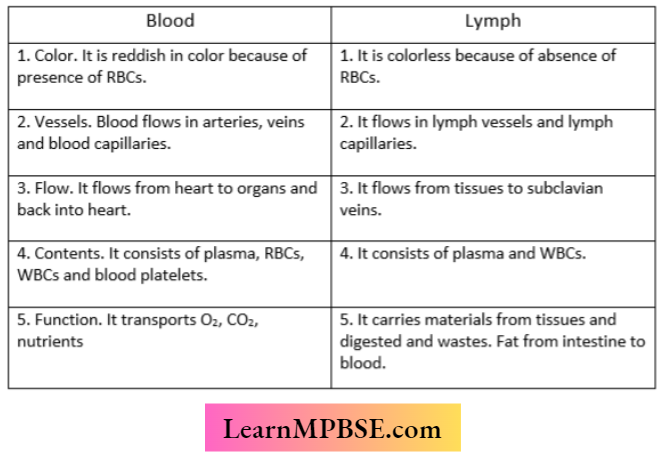
Question 9. List in tabular form three differences between blood and lymph.
Answer:
Question 10. “Blood circulation in fishes is different from the blood circulation in human beings.” Justify the statement.
Answer:
Fishes have two-chambered venous hearts with a single circulation. Human beings have four-chambered arterio¬venous hearts with double circulation.
Question 11. (1) Write the correct sequence of steps followed during the journey of oxygen-rich blood from the lungs to various organs of the human body.
(2) What happens when the system of blood vessels develops a leak?
Answer:
1. Lungs → Pulmonary veins → Left atrium → Left atrial diastole followed by contraction → Left ventricle → Left ventricle diastole followed by contraction → Aorta → Various parts of body except lungs.
2. (A) Leakage will reduce blood quantity, blood pressure mid-efficiency of a pumping system,
(B) Blood coagulation at the site of length will plug the leakage
MPBSE Class 10 Science Life Processes Question and Answers
Question 13. Give Reasons
Answer:
Question 1. At absolute zero
Answer: 1. Only para-hydrogen exists
Question 2. In which of the following reaction dihydrogen acts as an oxidising agent—
Answer: 4. 2Na + H2 → 2NaH
Question 3. Which of the following halogens has the least affinity towards hydrogen—
Answer: 1. I2
Question 4. Which of the following compounds on electrolysis produces hydrogen—
Answer: 3. Ba(OH)2 solution
MPBSE Class 11 Chemistry MCQs Question 5. The thermal stability of Gr.-15 hydrides follows the order
Answer: NH3 > PH3 > ASH3 > SbH3 > BiH3
Question 6. The correct order of vaporization enthalpy of the following hydride is
Answer: 3. PH3<AsH3<NH3
Question 7. Interstitial hydrides are formed by—
Answer: 3. D-block elements
Question 8. The correct descending order of thermal stability of alkali metals hydrides is—
Answer: 1. LiH > NaH > KH > RbH > CsH
MPBSE Class 11 Chemistry MCQs Question 9. Solubility of NaClin the solvents H20 and DaO is
Answer: 3. More in H20
Question 10. The degree of hardness of 1L sample water containing 0.002 mol MgS04 is
Answer: 2. 200 ppm
Question 11. Which of the following reacts with water to produce electron-precise hydrides—
Answer: 2. Al4C3
Hydrogen Class 11 MCQ Question 12. Which of the following couples reacts with water to produce the same gaseous product—
Answer: 2. K and K02
Question 13. Which of the following compounds contain free hydrogen
Answer: 3. Water gas
Question 14. Which of the following reacts with metallic sodium to produce hydrogen—
Answer: 4. C2H2
Question 15. Semi-water gas is
Answer: 1. CO + H2 + N2
Question 16. Which of the following metals does not react with cold water liberates H2 with boiling water
Answer: 4. Fe
Question 17. Volume of ’10 volume’ H202 required to convert 0.01 mol PbS into PbS04 is—
Answer: 4. 44.8 mL
Hydrogen Class 11 MCQ Question 18. On dilution of H202, the value of dielectric constant
Answer: 1. Increases
Question 19. By which of the following water gets oxidized to oxygen
Answer: 4. F2
Question 20. Which of the following does not get oxidized by H202
Answer: 4. O3
Question 21. The temperature at which the density of D20 is maximum is
Answer: 11.5°C
Question 22. Which of the following undergoes disproportionation reaction with water—
Answer: 2. F2
Hydrogen Class 11 MCQ Question 23. Which of the following undergoes disproportionation reaction with water—
Answer: 3. Cl2
Question 24. The non-inflammable hydrides
Answer: 1. NH3
Question 25. The triple point of water is
Answer: 3. 273K
Question 26. The process by which hydrogen is prepared by the reaction of silicon, iron alloy, and NaOH is
Answer: 3. Silicol process
Question 27. An element reacts with hydrogen to form a compound A, which in reaction with water liberates hydrogen again. The elements—
Answer: 2. CS
Question 28. Only one element of which of the following groups forms a metal hydride
Answer: 1. Gr-6
Question 29. An acidic solution of which of the following turns orange in the presence of H202 —
Answer: 3. Ti02
Question 30. In the following reaction the isotopic oxygens \(2 \mathrm{MnO}_4^{-}+3 \mathrm{H}_2 \mathrm{O}_2^{18} \rightarrow 2 \mathrm{MnO}_2+3 \mathrm{O}_2+2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{OH}^{-}\)
Answer: Both get converted into 02
Question 31. X on electrolysis produces Y which on vacuum distillation produces H202. The numbers of peroxo linkage present X and Y are
Answer: 3. 0>1
Question 32. The compound which on electrolysis in its molten or liquid state liberates hydrogen at the anode is
Answer: 2. CaH2
Question 33. Which of the following couples exhibit the maximum isotope effect
Answer: 1. H D
Question 34. Which of the following emits by tritium
Answer: 3. Particle
MPBSE Class 11 Chemistry MCQs Question 35. Oxidation of benzene by H202 in the presence of ferrous sulfate produces
Answer: 1. Pheno
Question 36. The oxidation state of Cr in the product obtained by the reduction of K2Cr20? by atomic hydrogen is
Answer: 4. +3
Question 37. Which of the following does not get reduced by H2 in its aqueous solution
Answer: 3. Zn2+
Question 38. Which of the following compounds has a similar odor as that of H202
Answer: 4. Nitric acid
Question 39. Which of the following compounds reacts with atomic hydrogen to form formaldehyde
Answer: 1. CO
Question 40. Which of the following isotopes of hydrogen is the most reactive
Answer: 1. H
MPBSE Class 11 Chemistry MCQs Question 41. When equal amounts of Zn are allowed to react separately with excess H2S04 and excess NaOH, then the ratio of the volumes of hydrogen produced for the first and the second case respectively
Answer: 4. 1:1
Question 42. Which of the following hydrides of s-block elements have a polymeric structure
Answer: 2. BeH2
Question 43. Which of the following statements is true—
Answer: 1. Ifz= 15, the element forms a covalent hydride
Question 44. Which of the following hydrides are polynuclear hydrides
Answer: C3H8
Question 45. Which of the following statements is correct
Answer: 1. Metalic hydrides are hydrogen deficient
Question 46. Which of the following ions get exchanged with Na+ ion of zeolite when zeolite is added to thehard water
Answer: 2. Ca2+ ion
MPBSE Class 11 Chemistry MCQs Question 47. Which of the following reactions are neurolysis
Answer: 2. AlClg + 3D20→ Al(OD)3 + 3DC1
Question 48. Which of the following reactions are redox reactions
Answer: 3. 2Na + 2H20 → 2NaOH + H2
Question 49. In which of the following reactions H202 acts as a reductant—
Answer: 3. Na0Br +H202 → NaBr + H20 + 02
Question 50. Which of the properties are the same for a metal and its hydride
Answer: 1. Hardness
Question 51. The correct orders are—
Answer: 1. H2 < D2 < T2 : boilingpoint
Question 52. Which of the following reacts with zinc to produce hydrogen gas
Answer: 2. cold water
Question 53. Which of the following properties has a greater magnitude in D20 than that in H20
Answer: 2. Viscosity
Question 54. Which of the following metal hydrides get reduced by hydrogen
Answer: 1. CuO
Question 55. Multimolecular covalent hydrides of s-block are
Answer: 2. BeH2
MPBSE Class 11 Chemistry MCQs Question 56. The oxidation numbers of the most electronegative element in the product were obtained due to the reaction between Ba02 and dil. H2S04 are—
Answer: 1. -1
Question 57. Which of the following compounds decreases the rate of decomposition of H202 —
Answer: 1. CO(NH2)2
Question 58. Which of the following produces H202 on hydrolysis
Answer: 1. Pemitricacid
Question 59. Choose the correct statements
Answer: 1. The Concentration of 20-volume H202 solution is 60.7g L-1
Question 60. Choose the correct alternative—
Answer: 1. a mixture of HC1 and HCIO is formed when chlorine reacts with cold water
Question 60. Which of the following alternatives is not true—
Answer: 1. Correct order of reactivity of h2 towards the halogens
Question 1. The amount of heat required to change the state of 1 kg of substance at constant temperature is called
Answer: 4. latent heat
Question 2. The water equivalent of a 400 g copper calorimeter (specific heat = 0.1 cal/gºC)
Answer: 1. 40 g
Question 3. Heat required to convert 1 g of ice at 0ºC into steam at 100ºC is
Answer: 3. 720 cal
Question 4. The thermal capacity of 40 g of aluminum (specific heat = 0.2 cal/gmºC)
Answer: 5. 8 cal/ºC
Class 11 Physics Thermal Expansion MCQs Question 5. The boiling water is changing into steam. Under this condition, the specific heat of water is
Answer: 3. Infinite
Question 6. One kg of ice at 0ºC is mixed with 1 kg of water at 10ºC. The resulting temperature will be
Answer: 2. 0ºC
Question 7. If 10g of ice at 0ºC is mixed with 10g of water at 40ºC, the final mass of water in the mixture is
Answer: 2. 15 g
Question 8. Water is used to cool the radiators of engines in cars because:
Answer: 2. Of its high specific heat
Question 9. Steam at 100ºC is passed into 2.0 kg of water contained in a calorimeter of water equivalent to 0.02 kg at 15ºC till the temperature of the calorimeter and its contents rise to 90ºC. The mass of steam condensed in kg is
Answer: 2. 0.280
Class 11 Physics Thermal Expansion MCQs Question 10. A small quantity, mass m, of water at a temperature θ (inºC) is poured onto a large mass M of ice which is at its melting point. Ιf c is the specific heat capacity of water and L is the latent heat of fusion of ice, then the mass of ice melted is given by :
Answer: 4. \(\frac{\mathrm{mc} \theta}{\mathrm{L}}\)
Question 11. 20 gm ice at –10 ºC is mixed with m gm steam at 100 ºC. Minimum value of m so that finally all ice and steam converts into water. (Use sice=0.5 cal/gmºC, swater=1 cal/gmºC,L (melting)=80 cal/gm and L (vaporization) = 540 cal/gm)
Answer: 1. \(\frac{85}{32} \mathrm{gm}\)
Question 12. 300 calories of heat is supplied to raise the temperature of 50 gm of air from 20°C to 30°C without any change in its volume. Change in internal energy per gram of air is
Answer: 4. 6.0 calories
Question 13. How much heat energy is gained when 5 kg of water at 20°C is brought to its boiling point? (Specific heat of water = 4.2 kg-1C-1)
Answer: 1. 1680 kJ
Question 14. Two rigid boxes containing different ideal gases are placed on a table. Box A contains one mole of nitrogen at temperature T0, While Box B contains one mole of helium at temperature (7/3) T0. The boxes are then put into thermal contact with each other and heat flows between them until the gases reach a common final temperature (ignore the heat capacity of boxes). Then, the final temperature of the gases, Tf, in terms of T0 is
Answer: 2. \(T_f=\frac{3}{2} T_0\)
Class 11 Physics Thermal Expansion MCQs Question 15. Compared to a burn due to water at 100°C, a burn due to stem at 100°C is
Answer: 1. More Dangerous
Question 16. Their moment should have its heat capacity small. If P is mercury thermometer, Q is resistance thermometer and R is thermocouple type then
Answer: 3. R is best, Q worst
Question 17. If 1g of steam is mixed with 1 g of ice, then the resultant temperature of the mixture is :
Answer: 3. 100°C
Question 18. The amount of heat required to convert a gram of ice at 0ºC into steam at 100ºC will be –
Answer: 1. 716 cal
Question 19. 10 grams of ice at 0ºC is mixed with 10 grams of water at 20ºC. The final temperature of the mixture will be
Answer: 3. 0ºC
Class 11 Physics Thermal Expansion MCQs Question 20. The amount of heat required to change 1 gm (0ºC) of ice into water of 100°C, is :
Answer: 3. 180 cal
Question 21. The latent heat of steam is 536 cal/gm, then its value in joule/kg is:
Answer: 1. 2.25 × 106
Question 22. At 100ºC, the substance that causes the most severe burn is –
Answer: 2. Steam
Question 23. The SI unit of the mechanical equivalent of heat is –
Answer: 2. Joule/calorie
Question 24. Volume expansion coefficient of a gas at constant pressure equal to:
Answer: 4. Inversely proportional to temperature
Question 25. 50 gm ice at 0ºC in an insulator vessel, 50 g water of 100ºC is mixed in it, then the final temperature of the mixture is (neglecting the heat loss) :
Answer: 1. 10ºC
Class 11 Physics Thermal Expansion MCQs Question 26. A bottle is filled with water at 30ºC. When it is taken on the moon then :
Answer: 4. Nothing will happen to the water
Question 27. Work done in converting one gram of ice at –10ºC into steam at 100ºC is –
Answer: 1. 3045 J
Question 28. The ratio of radii of two spheres of the same material is 1: 4, then the ratio of their heat capacities is
Answer: 4. \(\frac{1}{64}\)
Question 29. Heat given to a body which raises its temperature by 1°C is :
Answer: 2. Thermal capacity
Question 30. If mass-energy equivalence is taken into account, when water is cooled to form ice, in an isolated system the mass of water should:
Answer: 1. Increase
We have already seen that enthalpy is not the ultimate criterion of spontaneity. Another factor such as the randomness of the constituting particles (molecules, atoms, or ions) of the system may also be responsible for determining the spontaneity of a process.
Rudolf Clausius introduced a new thermodynamic property or state function known as entropy, from the Greek word ‘trope’ meaning transformation. It is denoted by the letter ‘S’. The entropy of a system is a measure of the randomness or disorderliness of its constituent particles.
The more disordered or random state of a system, the higher the entropy it has. Thus, from the molecular point of view, the entropy of a system can be defined below.
Class 11 Chemistry Notes For Entropy
The entropy of the system is a thermodynamic property that measures the randomness or disorderliness of the constituent particles making up the system.
According to the above definition, it may seem that entropy is related to the individual constituent particle of the system. However, thermodynamics, whose framework is based on a macroscopic approach, is not concerned with the existence and the nature of the constituent particles of the system.
Entropy, which is a macroscopic property, is in no way related to the behavior of the individual atoms or molecules of a system, instead, it reflects the average behavior of a large collection of atoms or molecules by which a system usually consists.
Since heat (q) is not a state function, the exchange of heat (5q) i.e., the amount of heat absorbed or rejected by a system during a process is not an exact differential.
But in a reversible process, the ratio of the heat exchanged between the system and surroundings \(\frac{\delta q_{r e v}}{T}\) to an absolute temperature at which the heat exchanger takes place is an exact differential. Hence, the quantity indicates the change ofa state function. This function is called entropy (S). Therefore, the change in entropy,
⇒ \(d S=\frac{\delta q_{r e v}}{T}=\frac{\begin{array}{c}
\text { Reversible heat transfer between } \\
\text { system and its surroundings }
\end{array}}{\begin{array}{c}
\text { Temperature (K) at which } \\
\text { heat is transferred }
\end{array}}\) …………………………….(1)
a reversible process, the state of a system changes from state 1 (initial state) to state 2 (final state), then the change in entropy (AS) in the process can be determined by integrating the equation
⇒ \(\int_1^2 d S=\int_1^2 \frac{\delta q_{r e v}}{T} \text { or, } S_2-S_1=\int_1^2 \frac{\delta q_{r e v}}{T} \text { or, } \Delta S=\int_1^2 \frac{\delta \tilde{q} q_{r e t}}{T}\)
For the process occurring at a constant temperature, the change in entropy, \([\Delta S=\frac{1}{T} \int_1^2 \delta q_{r e v}=\frac{q_{r e v}}{T}.\)
Class 11 Chemistry Notes For Entropy
It is not possible to define entropy; however, we can define the change in entropy of a system ( dS or AS) undergoing a reversible process. It is defined as the ratio of reversible heat exchange between the system and its surroundings to the temperature at which the heat exchange takes place.
The relation tells us for a given input of heat into a system, the entropy of the system increases more at a lower temperature than at a higher temperature.
The randomness in a system is a measure of its entropy. The more randomness the more entropy. Therefore, for a given input of heat into a system, the randomness of the system increases more when heat is added to the system at a lower temperature than at a higher temperature.
The entropy of a system quantifies the unpredictability of its constituent particles. Entropy is a state function as its value for a system relies solely on the current state of the system, and its change (ΔS) during a process is determined exclusively by the beginning and final states of the system, independent of the pathway taken to execute the process.
There exists a relationship between the entropy of the system and the randomness of its constituent particles (atoms, ions, or molecules).
The entropy of a system increases or decreases with the increase or decrease in randomness of the particles constituting the system. Therefore, entropy is the measure of the randomness of the constituent particles in a system. this is the physical significance of entropy.
Class 11 Chemistry Notes For Entropy
The change in entropy is defined in terms of a reversible process, for which it is defined as
⇒ \(d S=\frac{\delta q_{r e v}}{T}\) where 8qrev) Is the reversible exchange of heat between a system and its surroundings at 7’K. in case of an irreversible process, the change in entropy
⇒ \(d S \neq \frac{\delta q_{i r r}}{T}\) where 5<](rr represents irreversible exchange of heat between a system and its surroundings at 7’K.
As entropy is a state function, its change in a particular process does not depend on the nature of the process. Thus, the change in entropy in a process carried out reversibly is the same as the change in entropy that occurs if the same process is carried out irreversibly.
In the CGS system, the unit of entropy = cal.deg-1, while the unit of entropy in SI = J. K-1
Change In Entropy Of The System In Some Processes
When a system undergoes a process, its change in entropy in the process is ΔS = S2 – S1; where S1 and S2 are the entropies of the initial and final states of the system respectively, in a process.
In a process, if S2 > S1, then ΔS is positive. This means that the entropy of a system increases in the process. For example, the melting of ice or vaporization of water is associated with an increase in the entropy of the system, so AS is involved in these processes.
If S2 < S1, then ΔS is negative, indicating that the entropy of the system decreases in the process. For example, when ice is formed from liquid water or water is formed from water vapor, the entropy of the system decreases i.e., ΔS =-ve.
In any chemical reaction, the initial entropy (S1) of the system means the total entropy of the reactants, and the final entropy of the system (S2) means the total entropy of the products.
Hence, the change in entropy in a chemical reaction, ΔS = \(S_2-S_1^{3 i}=\sum S_{\text {products }}-\sum S_{\text {reactants }} \text {, where, } \sum S_{\text {reactants }}\text { and } \sum S_{\text {products }}\)
Change in entropy of the system in a cyclic process:
The change in entropy of the system in any process, ΔS = S2– S1 where S1 and S2 are the initial and final entropies of the system, respectively. Because entropy is a state function, and in a cyclic process the initial and the final states of a system are the same, = S2, and the change in entropy ofthe system, ΔS = 0.
In an adiabatic process, heat exchange does not occur between a system and its surroundings. Therefore, in a reversible adiabatic process, qrev = 0 and the change in entropy ofthe system in this process.
⇒ \(d S=\frac{\delta q_{r e v}}{T}=\frac{0}{T}=\mathbf{0} \text { or, } d s=0 \text { or, } \Delta S=0\)
Therefore, the change in entropy of a system undergoing a reversible adiabatic process is zero, i.e., the entropy of a system remains the same in an adiabatic reversible change. Owing to this a reversible adiabatic process is sometimes called an isentropic process.
Class 11 Chemistry Notes For Entropy
Change in entropy of the system in an irreversible adiabatic process: Like reversible adiabatic process, heat exchange does not also occur in an irreversible adiabatic process. However, it can be shown that in an irreversible adiabatic process, the entropy change of a system is always positive, i.e., ΔS > 0.
Let us consider, a system change from state 1 to state 2 in an isothermal reversible process. Therefore, in this process, the change in entropy of the system.
⇒ \(\int_1^2 d S=\int_1^2 \frac{\delta q_{r e v}}{T} \text { or, } S_2-S_1=\frac{1}{T} \int_1^2 \delta q_{r e v} \text { or, } \Delta S=\frac{q_{r e v}}{T}\)
Since T= constant as the process is isothermal]
Melting of a solid, vaporization of a liquid, solidification of a liquid, condensation of vapor, etc. are some examples of phase transition.
At a particular temperature, a phase transition occurs at a constant pressure. The temperature remains unaltered during the transition although heat is exchanged between the system and the surroundings.
Class 11 Chemistry Notes For Entropy
The phase transition can be considered as a reversible process. If qrev of heat is absorbed during a phase transition at constant pressure and 7’K, then the change in entropy, of the system. As the process is occurring at constant pressure
⇒ \(q_{\text {re }}=q_p=\Delta H.\).
hence \(\Delta s=\frac{\Delta n}{r}\) \(\Delta s=\frac{\Delta n}{r}\) ……………………….(1)
It In defined as the change in entropy associated with the transformation of one mole of a solid substance into its liquid phase at its melting point
According to equation (1) \(\Delta S_{f u s}=\frac{\Delta H_{f u s}}{T_f}\)
where ΔHvap= the enthalpy of fusion = die heat required for the transformation of 1 mol of a solid at its melting point into 1 ml of liquid and T1= melting point (K) of the given solid As the fusion of a solid substance is an endothermic process (Le, A> 0 ), the change in entropy due to fusion (ΔSvap) is always positive.
Class 11 Chemistry Notes For Entropy
In the solid phase of a substance, the constituent particles are held in an ordered state. The degree of orderliness is less in the liquid phase as the particles in the liquid have freedom of motion. This is why, when a solid melts, the randomness within the system increases, causing an increase in the entropy of the system.
Example: The enthalpy of fusion of ice at 0°C and 1 atm. Therefore, the change in entropy during the transformation of 1 mol of ice into 1 mol of water at 0°C and 1 atm is-
It is defined as the change in entropy when one mole of a liquid at its boiling point changes to its vapor phase.
Where ΔH = the enthalpy of vaporization = the heat required for the transformation of 1 mol of liquid at its boiling point into 1 mol of vapor and Tb = boiling point ofthe liquid (K).
As the vaporization of a liquid is an endothermic process [i.e., \(\Delta H_{v a p}>0\)), the change in entropy in a vaporization process (ΔSvap) is always positive. When a liquid vaporizes, the molecular randomness in the system increases as the molecules in the vapor phase have more freedom of motion thus they have in the liquid phase. As a result, the vaporization of a liquid always leads increase in the entropy ofthe system.
Example: The enthalpy of vaporization of water at 100°C and 1 atm (ΔHvap) = 40.4 kj .mol-1
. Thus, the change in entropy due to the transformation of 1 mol of water into 1 mol of water vapor at 0°C temperature and 1 atm pressure is
⇒ \(\Delta S_{\text {vap }}=\frac{\Delta H_{\text {vap }}}{T_b}=\frac{40.4 \mathrm{~kJ} \cdot \mathrm{mol}^{-1}}{373 \mathrm{~K}}=108.3 \mathrm{~J} \cdot \mathrm{K}^{-1} \cdot \mathrm{mol}^{-1}\)
Class 11 Chemistry Notes For Entropy
Entropy change in an isothermal reversible expansion or compression of an ideal gas
Let, n mol of an ideal gas undergoes an isothermal reversible expansion from its initial state (P1 V1 to the final state (P2, V2). The equation showing the change in entropy of the gas in the process can be derived.
The result of this derivation gives the relation—
⇒ \(\Delta S=n R \ln \frac{V_2}{V_1}=2.303 n R \log \frac{V_2}{V_1}=2.303 n R \log \frac{P_1}{P_2}\)
As the gas expands, V2 > V2 (or, P1> P2 ), so according to the equation [1], the change in entropy (AS) of the gas due to its expansion is positive i.e., the entropy of the system increases.
If the isothermal reversible compression of the same amount of gas causes a change in the state of the gas form (P1 V1 to (P2, V2), then the change in entropy of the gas is given by
⇒ \(\Delta S=n R \ln \frac{V_2}{V_1}=2.303 n R \log \frac{V_2}{V_1}=2.303 n R \log \frac{P_1}{P_2}\)
As the gas is compressed, V2 < V1 (or P2> P1 ), According to equation [2], the change in entropy (AS) of the gas due to its compression is negative, i.e., the entropy of the system decreases.
Class 11 Chemistry Notes For Entropy
If the volume of a gas is increased, the gas molecules will get more space for their movement i.e., the gas molecules will move in greater volume. As a result, the randomness of the gas molecules as well as the entropy ofthe system (gas) will increase.
Thus, the entropy of a gas increases with the increase of its volume. On the other hand, the entropy of a gas decreases with the decrease of its volume.
When a system exchanges heat with its surroundings, the entropy of the system as well as its surroundings changes.
To calculate the change in entropy of the surroundings, the given points are to be considered:
Surroundings are so large compared to one system that they serve as a heat reservoir without undergoing any temperature change.
Surroundings absorb or release heat reversibly, and during these processes, the temperature and pressure of the surroundings remain almost the same In a process at TK, if the amount of heat released by the system to the surroundings is sys, then the amount of heat absorbed by tire surroundings = -guys (the sign of q is -ve).
Therefore, in this process, the change in entropy of the surroundings \(\Delta S_{s u r r}=-\frac{q_{s y s}}{T}\)
Class 11 Chemistry Notes For Entropy
Hence, the entropy of the surroundings increases if heat is released by the system to the surroundings.
In a process at T K, if the amount of heat absorbed by the system from the surroundings is q, then the amount of heat released by the surroundings will be -qsys (the sign of qsys is +ve ). So, in this process, the change in entropy of the surroundings, \(\Delta S_{\text {surr }}=-\frac{q_{s y s}}{T}.\)
Hence, the entropy of the surroundings decreases if heat is absorbed by the system from the surroundings
Standard molar entropy of a substance:
Entropy of 1 mol of a pure substance at a given temperature (usually 25°C) &1 atm pressure is termed as the standard molar entropy of that substance.
It is denoted by S° and its unit is J. K-1.mol-1.
Standard entropy change in a chemical reaction (ΔS°):
In a chemical reaction, the change in standard entropy, ΔS° = total standard entropies of the products – total standard entropies of the reactants i.e.,
⇒ \(\Delta s^0=\sum n_i s_i^0-\sum n_j s_j^0\)
Where Soi and Soj are the standard entropy of the i -tit product and j -th reactant, respectively. n1 and n2 are the number of moles of the Mil product and i-th reactant, respectively In the balanced equation.
Class 11 Chemistry Notes For Entropy
In the case of the reaction.
⇒ \(a A+b B \rightarrow c C+a D\Delta S^0=\left(c S_C^0+d S_b^0\right)-\left(a S_A^0+b S_B^0\right)\)
The change In entropy of the surroundings in a given process,
⇒ \(\Delta S_{s t u r}=\frac{-q_{s y s}}{T},\) where qÿ is the heat absorbed by the system at 7’K.
In case of chemical reactions occurring at constant pressure \(q_{s y s}=q_P=\) change in enthalpy of the reaction system
⇒\(=\Delta H . \mathrm{So}_1, \Delta \mathrm{S}_{\text {surr }}=-\frac{\Delta H}{T}\)
For exothermic reactions,
⇒ \(\Delta H<0. \text { So, } \Delta S_{\text {surr }}=+v e \text {. }\) I-Ience, the entropy of the surroundings increases In an exothermic reaction.
For endothermic reaction
⇒ \(\Delta H>0 \text {. So, } \Delta S_{\text {surr }}=-v e \text {. }\) Hence, the entropy of the surroundings decreases in an endothermic reaction.
Thermodynamic Process Definition:
A system is said to undergo thermodynamics. A pathway of process: The sequence of system a system process when it undergoes a processis called the pathways of process
Cyclic process Definition
A system is said to haw undergone a cyclic process I if it returns to Its initial state after a series of successive
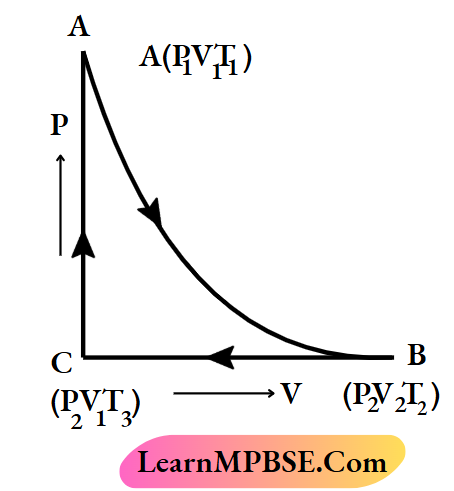
Cyclic process Explanation:
Let us consider a process, in which the initial state of a gaseous system is A (Pv Tx). The system returns to its initial state after undergoing consecutive returns to its initial state through three successive operations, it is a cyclic process.
Class 11 Chemistry Notes For Thermodynamic Process
Cyclic process Example:
The following change indicates a cyclic process because 1 mol of water (system) returns to its initial state again through successive changes
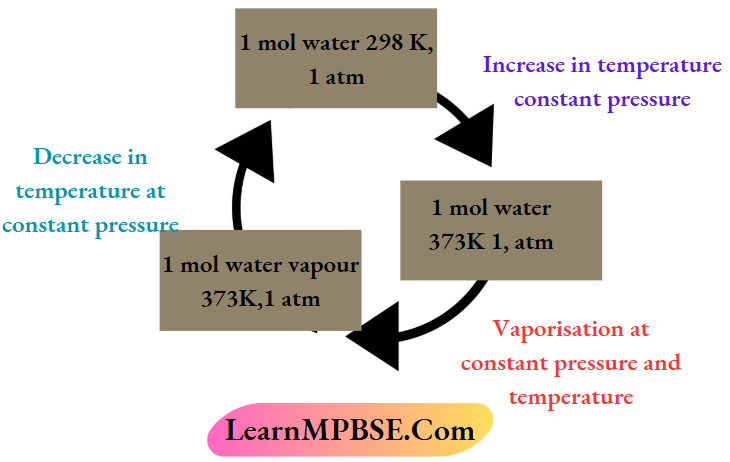
The changes in state functions are zero in a cyclic process. The value of a state function depends upon the present state of the system. Since the initial and the final states of the system are the same in a cyclic process, the rallies of the state functions in these two states are also the same. So die change in state function (ΔP, ΔV,ΔT,ΔU’, ΔH, etc.) becomes zero for a cyclic process
Isothermal process Definition
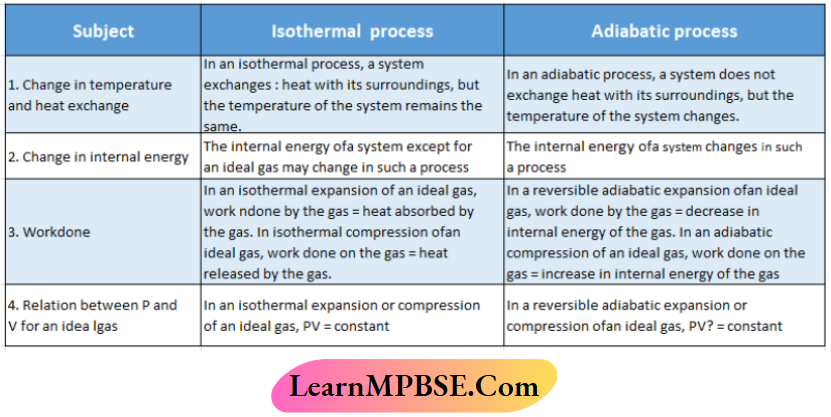
If the temperature of a thermodynamic system remains constant throughout a process, then the process Is said to be an Isothermal process,
At the time of conducting this process, the system Is kept lit contact with «a constant temperature heal hath (f.u, thermostat) with a high heat capacity. Such a heat hath Is capable of gaining or losing heat without changing Its temperature.
Condition(s) for the isothermal process:
During an Isothermal process, the temperature of the system remains constant. So we can write,dT ‘[system] = constant and d’V[system] or ΔT [system] = 0.
Class 11 Chemistry Notes For Thermodynamic Process
Condition(s) for the isothermal process Example:
The boiling of a liquid at its boiling point Is an isothermal process. This Is because the temperature of the liquid remains constant until the entire liquid converts to vapor. Thus, the boiling ofwater at 100°C and l atm is an isothermal process.
The temperature of the system remains fixed in the isothermal process. U does not mean that heat is not absorbed or liberated by the system during this process.
Isobaric process Definition
A process in which the pressure of the system remains fixed at each step of the process is called an isobaric process.
Condition(s)t for this and the isobaric process:
As the pressure of the system during this process remains constant P{system)=constant and dp (system)or ΔP (system) =0
Isobaric process Example:
The vaporization of any liquid in an open container occurs under atmospheric pressure. If the atmospheric pressure remains fixed, then the process of vaporization is said to be an isobaric process
Isochoric process Definition:
The isochoric process in Which the volume of the system remains constant throughout the process is called an isochoric to process.
Condition(s) for the isochoric process:
The volume of the system remains constant during this process. Hence, V[system] = constant and dV[system] or ΔV [system] =0
Isochoric process Example: The combustion of n substance In a bomb calorimeter.
For a closed system consisting of an ideal gas. the plots of P vs V for
These are given below:
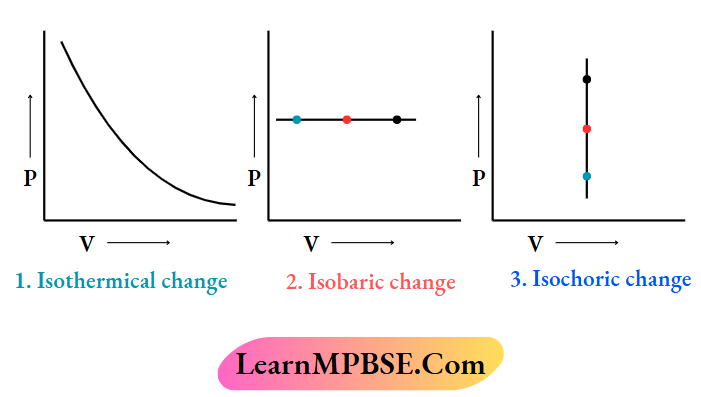
Class 11 Chemistry Notes For Thermodynamic Process
Adiabatic process Definition
A process in which no exchange of heat takes place between the system and Its surroundings at any stage of the process is called an adiabatic process.
This process requires the system to be covered with a perfect thermal insulator. But in reality, no such material is available and hence the process never becomes one hundred percent adiabatic.
Condition(s) for adiabatic process:
No heat is exchanged between a system and its surroundings In an adiabatic process. So, for such type of process q – 0; where q = heat absorbed or released by the system.
Adiabatic process Example:
The sudden expansion or compression of a gas is considered to be an adiabatic process because when a gas (system) undergoes such a process, it does not get a chance to exchange heat with its surroundings. As a result, the sudden compression of a gas results in an increase in the temperature of the gas, while its sudden expansion leads to a decrease in temperature. For example, when the valve ofa bicycle or car tire is removed, the air coming out ofthe tire undergoes very fast expansion and gets cold.
Reversible process Definition
A process It salt! to be reversible If It Is carried out Infinitesimally slowly so that In each step the thermodynamic equilibrium of the system Is maintained, and any Infinitesimal change In conditions can reverse the process to restore both the system and Its surroundings lo their Initial states.
Class 11 Chemistry Notes For Thermodynamic Process
Reversible process Explanation:
Let us consider that the gas (system) Is enclosed lit a cylinder lilted with a weightless and frictionless piston and the cylinder is kept At a constant temperature hath (surroundings). So, any change occurring In the ire system would be Isothermal. Since the temperature of the system and surroundings tiro the same, the system will remain In thermal equilibrium.
Let the applied external pressure acting on the piston Is the same as that of the gas. So, the system will remain In mechanical equilibrium. Hence, In this condition, the system, the gas Is In a state of thermodynamic equilibrium and pthe roperties of the system remain unchanged
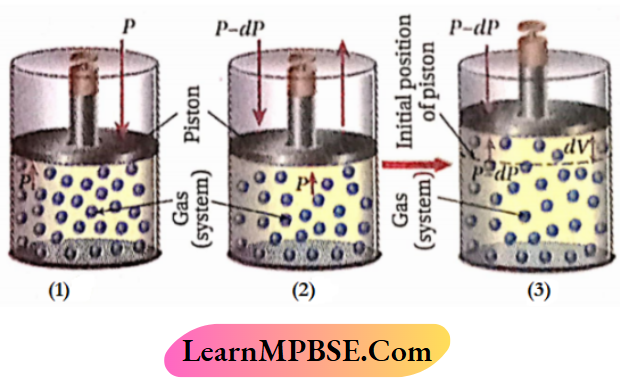
Now, if the external pressure is decreased by an infinitesimal amount dP, the volume of the gas will increase very slowly by an infinitesimal amount until the pressure of the gas becomes equal to that of the external pressure.
Class 11 Chemistry Notes For Thermodynamic Process
Characteristics of a reversible process:
Examples of some reversible processes:
1. The vaporization ofa liquid at a particular temperature in a closed container can be considered as a reversible process. Let us consider a certain amount of water is in equilibrium with its vapour at T K temperature in a closed container. Here water and water vapour together constitute a system.
2. Reaction occuring in a galvanic cell is reversible in nature.
Class 11 Chemistry Notes For Thermodynamic Process
Irreversible process Definition
Aprocess which occurs at a finite rate with the finite ite changes in properties of the system, and at any stage during the process, the system cannot get a chance to remain in thermodynamica equilibrium is called an irreversible process
All natural processes are irreversible in nature.
Irreversible process Explanation:
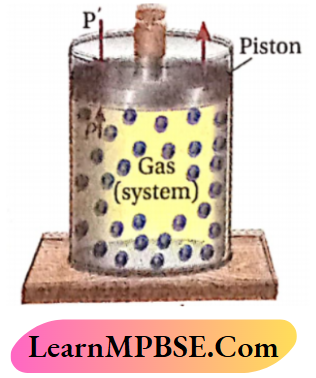
Let us consider that a certain amount of a gas is enclosed in a cylinder fitted with a weightless and friction¬less piston and the cylinder is kept in a constant temperature heat-bath (thermostat).
Characteristics of an irreversible process:
In an irreversible process there is an appreciable difference between the driving force and the opposing force (actually, the driving force is greater than the opposing force).
Irreversible process Examples: The flow of heat from an object of higher temperature to an object of lower temperature.
Class 11 Chemistry Notes For Thermodynamic Process
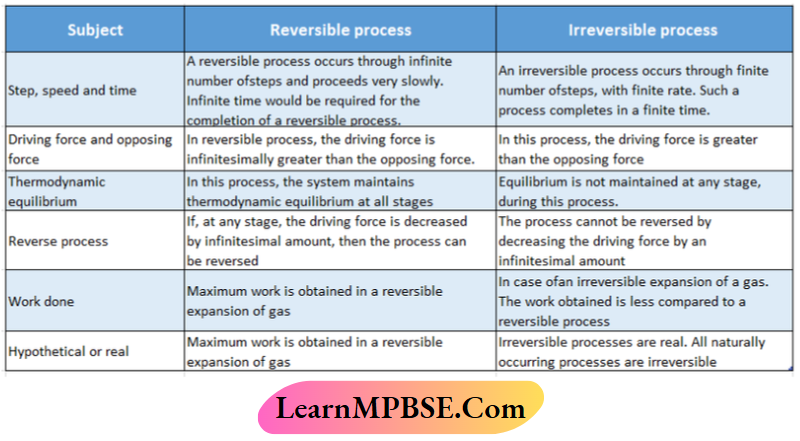
Differences between the reversible and irreversible processes:
Differences between the reversible and irreversible processes: