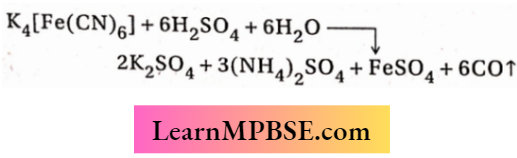MPBSE Class 11 Chemistry Chemical Properties Of Hydrogen
Question 1. Write the names of isotopes of hydrogen. What is the mass ratio of these isotopes?
Answer: In nature, there are three isotopes of hydrogen. These are protium \(\left({ }_1^1 \mathrm{H}\right) \text {, deuterium }\left[{ }_1^2 \mathrm{H} \text { or } \mathrm{D}\right] \text { and tritium }\left[{ }_1^3 \mathrm{H} \text { or } \mathrm{T}\right] \text {. }\)
The mass ratio of \({ }_1^1 \mathrm{H},{ }_1^2 \mathrm{H} \text { and }{ }_1^3 \mathrm{H} \text { is } 1: 2: 3 \text {. }\)
Question 2. Why does hydrogen occur in a diatomic form rather than in a monoatomic form under normal conditions?
Answer: A hydrogen atom has only one electron and thus, it has one electron less than the stable configuration of the nearest noble gas helium. Thus, to achieve a stable configuration it shares its single electron with the electron of another H -atom to form a stable diatomic molecule (H2 ).
Read and Learn More Class 11 Chemistry
Question 3. Complete the following reactions:
- \(\mathrm{H}_2(g)+\mathbf{M}_m \mathrm{O}_{\mathrm{o}}(s) \stackrel{\Delta}{\longrightarrow}\)
- \(\mathrm{CO}(g)+\mathrm{H}_2(g) \frac{\Delta}{\text { catalyst }}\)
- \(\mathrm{C}_3 \mathrm{H}_8(g)+3 \mathrm{H}_2 \mathrm{O}(g) \underset{\text { catalyst }}{\longrightarrow}\)
- \(\mathrm{Zn}(s)+2 \mathrm{NaOH}(a q) \stackrel{\Delta}{\longrightarrow}\)
Answer:
- \(o\mathrm{H}_2(g)+\mathrm{M}_m\mathrm{O}_o(s)\stackrel{\Delta}{\longrightarrow}m\mathrm{M}(s)+o\mathrm{H}_2\mathrm{O}(l)\)
- \(\mathrm{CO}(g)+2 \mathrm{H}_2(g) \underset{\text { catalyst }}{\longrightarrow} \mathrm{CH}_3 \mathrm{OH}(l)\)
- \(\mathrm{C}_3 \mathrm{H}_8(\mathrm{~g})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \stackrel{\mathrm{Ni}, 1270 \mathrm{~K}}{\longrightarrow} 3 \mathrm{CO}(g)+7 \mathrm{H}_2(g)\)
- \(\mathrm{Zn}(s)+2 \mathrm{NaOH}(a q) \stackrel{\Delta}{\longrightarrow} \underset{\text { Sodium zincate }}{\mathrm{Na}_2 \mathrm{ZnO}_2(a q)+\mathrm{H}_2(g)}\)
Class 11 Chemistry Chemical Properties Of Hydrogen
Question 4. Discuss the consequences of high enthalpy of the H —H bond in terms of the chemical reactivity of dihydrogen.
Answer: H —H bond length is very small (74 pm) because the H-atom has a very small atomic size. Consequently, H—H bond dissociation enthalpy is very high (435.9 kJ-mol-1) which makes hydrogen completely inert at ordinary temperature. However, at higher temperatures, the H—H bond undergoes dissociation in the presence of a catalyst to form hydrides with metals and non-metals.
Question 5. What characteristics do you expect from an electron-deficient hydride concerning its structure and chemical reactions?
Answer:
- There is an insufficient number of electrons in the valence shell of the central atom. So, in this type of hydride, the valence shell of the central atom does not have a complete octet configuration. For example, in BH3, the valence shell of B has six electrons and the hydrides are trigonal planar shape.
- Due to electron deficiency, this type of hydrides act as Lewis acids, i.e., they accept electron pairs. For example,\(\mathrm{H}_3 \mathrm{~B}+\mathrm{NH}_3 \rightarrow \mathrm{H}_3 \mathrm{~B} \leftarrow: \mathrm{NH}_3\)
- To compensate for the electron deficiency, the hydrides form dimers, trimers, and polymers and attain stability. for example B2H6,B4H10,(AIH3)n etc.
- Electron-deficient hydrides are extremely reactive. They easily react with both metals and non-metals.
Question 6. Do you expect the carbon hydrides of the (CnH2n+2) to act as ‘Lewis’ acid or base? Justify your answer.
Answer: The hydrides of carbon of the type CnH2n+2 electron-precise hydrides, i.e., they have an exact number of electrons in the valence shell of the central atom so as to write their conventional Lewis structures. Therefore, they do not have a tendency to either gain or lose electrons and consequently, they do not act as Lewis acids or bases.
Question 7. What do you understand by the term “nonstoichiometric hydrides”? Do you expect this type of hydride to be formed by alkali metals? Justify your answer.
Answer:
Hydrides of d and f-block elements that are low in hydrogen, characterized by a fractional metal-to-hydrogen ratio, are termed non-stoichiometric hydrides.
- The alkali metals, due to their strong reducing properties, donate their lone pair of electrons to the hydrogen atom, resulting in the formation of hydride ions.
- As a hydride ion H- is generated through the complete transfer of an electron, the ratio of metal to hydrogen remains constant in these hydrides, resulting in compositions that reflect a straightforward whole-number ratio. Consequently, the alkali metals only produce stoichiometric hydrides.
Chemical Properties Of Hydrogen
Question 8. How do you expect the metallic hydrides to be useful for hydrogen storage? Explain.
Answer:
- Certain transition metals, such as palladium (Pd) and platinum (Pt), adsorb significant quantities of hydrogen atoms on their surfaces, resulting in the formation of hydrides.
- The incorporation of hydrogen atoms causes the metal lattice to expand, resulting in decreased stability.
- Consequently, upon heating, these metal hydrides breakdown to liberate dihydrogen and revert to a finely split metallic state. The dihydrogen produced in this method can serve as a fuel.
- Consequently, transition metals or their alloys can be utilized for the storage and delivery of hydrogen as a fuel.
Question 9. Among NH3, and H2O midIIF, which would you expect to have the highest magnitude of hydrogen bonding & why?
Answer:
The strength of a hydrogen bond depends upon the atomic size mid the electronegativity of the atom to which the H-atom Is covalently linked. Smaller size and higher electronegativity of the other atom favor the formation of stronger H -bonding and that Is due to increase In magnitude of bond polarity. Among N, F, and O atoms, V has the lowest atomic size and the highest electronegativity. Hence the II — F bond is maximum polar and as a result, it will have the highest magnitude of H-bonding.
Question 10. Saline hydrides are known to react with water violently producing fire. Can CO2, a well-known fire extinguisher, be used in this case? Explain.
Answer: The saline hydrides (For example, NaH, CaH2, etc.), react with water violently to yield the corresponding metal hydroxides with the evolution of H2 gas. The liberated H2 gas undergoes spontaneous combustion causing fire and this is because of the highly exothermic nature of the combustion reaction.
⇒ \(\mathrm{NaH}(s)+\mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{NaOH}(a q)+\mathrm{H}_2(g) ;\)
⇒ \(2 \mathrm{H}_2+\mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}(l) ; \Delta H^0=-286 \mathrm{~kJ} \cdot \mathrm{mol}^{-1}\)
In this case, CO2 cannot used as a fire extinguisher because its gels are reduced by the hot metal hydride to form formate ions.
⇒ \(\stackrel{-1}{\mathrm{NaH}}+\stackrel{+4}{\mathrm{CO}_2} \rightarrow \stackrel{+1+2}{\mathrm{H} C O O N a}\)
Question 11. Arrange the following:
- CaH2, BeH2, and TiH2 in order of increasing electrical conductance.
- LiH, NaH, and CsH in order of increasing ionic character.
- If —H, D—D, and F—F in order of increasing bond dissociation enthalpy.
- NaH, MgH2, and H2O in order of Increasing and reducing properties.
Answer:
- Being a covalent hydride BeH2 does not conduct electricity at all. Being an Ionic hydride CaH2 conducts electricity in the fused state while TiH2, being a metallic hydride, conducts electricity at room temperature, ‘t hus, the order of increasing electrical conductance is: BeH2 < CaH2 < TiH2.
- The electronegativity of the alkali metals decreases down the group from Li to Cs. Therefore, the ionic character of their hydrides also increases in the same order, l.e., LiH < NaH < CsH.
- The bond dissociation enthalpy of the: F—F: bond is the lowest (242.6 kJ • mol-1) and this is due to the high concentration of electron density around each F atom in the form of three unshared pairs which significant repulsive interactions. Again, because of the marginally smaller size of D as compared to H, the bond dissociation enthalpy of the D—D bond (443.35 kJ-mol-1) is slightly higher than that of the H —H bond (435.88 kJ-mol-1). Hence, the bond dissociation enthalpy increases in the order: of F —F < H —H < D —D.
- NaH, being an ionic hydride, is a more powerful reducing agent than the covalent hydrides MgH2 and H2O. MgH2 is a stronger reducing agent than H20 because the bond dissociation enthalpy of the Mg—H bond is much lower than that of the O —H bond. Therefore, the reducing property increases in the order: of H2O < MgH2 < NaH.
Class 11 Chemistry Chemical Properties Of Hydrogen
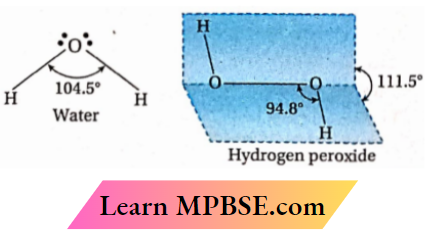
Question 12. Compare the structures of H20 and H202.
Answer: The oxygen atom in water is sp3 -sp3-hybridized. The two O —H bonds are sp3-s sigma bonds. The H —O —H bond angle is 104.5°. This value is a little less than the tetrahedral angle (109°28/) because of stronger lone pair-lone pair and lone pair-bond pair repulsions than bond pair-bond pair repulsion. Thus, water is a bent molecule. Each oxygen atom in H2O2 is also sp3 hybridised. The O —0 bond is a sp3-sp3 sigma bond and the two O —H bonds are sp3-s sigma bonds. The two O —H bonds are, however, present in different planes. In the gas phase, the dihedral angle between the two planes (i.e., the planes containing H —O —O system) is 111.5°. So, the molecule has an open-booklike structure.
Question 13. What do you understand by the term ‘auto-protolysis’ of water? What is its significance?
Answer: Self-ionization of water is called auto-protolysis. Self-ionization of water can be expressed by the given equation
⇒ \(\begin{aligned} & \mathrm{H}_2 \mathrm{O}(l)+\mathrm{H}_2 \mathrm{O}(l) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(a q)+\mathrm{OH}^{-}(a q) \\ & \text { Acid-1 Base-2 } \quad \text { Acid-2 } \quad \text { Base-1 } \\ & \end{aligned}\)
Water exhibits amphoteric properties because of auto-protolysis. Thus water reacts with both acids and bases. It usually acts as a base in the presence of an acid stronger than it and acts as an acid in the presence of a base stronger than it. For example,
⇒ \(\begin{aligned} & \mathrm{H}_2 \mathrm{O}(l)+\mathrm{NH}_3(a q) \longrightarrow \mathrm{NH}_4^{+}(a q)+\mathrm{OH}^{-}(a q) \\ & \begin{array}{llll} \text { Acid-1 } & \text { Base-2 } & \text { Acid-2 } & \text { Base-1 } \end{array} \\ & \end{aligned}\)
⇒ \(\begin{aligned} & \mathrm{H}_2 \mathrm{O}(l)+\mathrm{HCl}(a q) \longrightarrow \mathrm{H}_3 \mathrm{O}^{+}(a q)+\mathrm{Cl}^{-}(a q) \\ & \text { Base-1 } \quad \text { Acid-2 } \quad \text { Acid-1 } \quad \text { Base-2 } \\ & \end{aligned}\)
Question 14. Consider the reaction of water with F2 and suggest, In terms of oxidation and reduction, which species are oxidized/reduced.
Answer:
⇒ \( 2 \mathrm{~F}_2(g)+2 \mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{O}_2(g)+4 \mathrm{H}^{+}(a q)+4 \mathrm{~F}^{-}(a q) Oxidant Reductant\)
⇒ \( 3 \mathrm{~F}_2(g)+3 \mathrm{H}_2 \mathrm{O}(g) \rightarrow \mathrm{O}_3(g)+6 \mathrm{H}^{+}(a q)+6 \mathrm{~F}^{-}(a q) Oxidant Reductant\)
In these reactions, water acts as a reductant and itself gets oxidized to oxygen or ozone. In this case, highly electronegative fluorine acts as an oxidant and gets reduced to F-.
Question 15. Complete the following chemical reactions.
- \(\mathrm{PbS}(s)+\mathrm{H}_2 \mathrm{O}_2(a q) \rightarrow\)
- \(\mathrm{MnO}_4^{-}(a q)+\mathrm{H}_2 \mathrm{O}_2(a q) \rightarrow\)
- \(\mathrm{CaO}(s)+\mathrm{H}_2 \mathrm{O}(g) \rightarrow\)
- \(\mathrm{AlCl}_3(g)+\mathrm{H}_2 \mathrm{O}(l) \rightarrow\)
- \(\mathrm{Ca}_3 \mathrm{~N}_2(s)+\mathrm{H}_2 \mathrm{O}(l) \rightarrow\)
Classify the above into [a] hydrolysis, [b] redox, and [c] hydration reactions.
Answer:
⇒ \(\mathrm{PbS}(s)+4 \mathrm{H}_2 \mathrm{O}_2(a q) \longrightarrow \mathrm{PbSO}_4(s)+4 \mathrm{H}_2 \mathrm{O}(l)\)
⇒ \(\begin{array}{r}
2 \mathrm{MnO}_4^{-}(a q)+5 \mathrm{H}_2 \mathrm{O}_2(l)+6 \mathrm{H}^{+}(a q) \longrightarrow \\
2 \mathrm{Mn}^{2+}(a q)+8 \mathrm{H}_2 \mathrm{O}(l)+5 \mathrm{O}_2(g)
\end{array}\)
⇒ \(\mathrm{CaO}(s)+\mathrm{H}_2 \mathrm{O}(g) \longrightarrow \mathrm{Ca}(\mathrm{OH})_2(a q)\)
⇒ \(\begin{array}{r}
\mathrm{AlCl}_3(g)+6 \mathrm{H}_2 \mathrm{O}(l) \longrightarrow \\
{\left[\mathrm{Al}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}(a q)+3 \mathrm{Cl}^{-}(a q)}
\end{array}\)
⇒ \(\begin{array}{r}
{\left[\mathrm{Al}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}(a q)+\mathrm{H}_2 \mathrm{O}(l) \longrightarrow} \\
{\left[\mathrm{Al}\left(\mathrm{H}_2 \mathrm{O}\right)_5(\mathrm{OH})\right]^{2+}(a q)+\mathrm{H}_3 \mathrm{O}^{+}(a q)}
\end{array}\)
⇒ \(\mathrm{Ca}_3 \mathrm{~N}_2(s)+6 \mathrm{H}_2 \mathrm{O}(l) \rightarrow 3 \mathrm{Ca}(\mathrm{OH})_2(a q)+2 \mathrm{NH}_3(a q)\)
Reactions 1 and 2 are redox reactions. Reactions 3 and 4 are hydrolysis reactions. Reaction 5 is the hydration reaction.
Class 11 Chemistry Chemical Properties Of Hydrogen
Question 16. Is demineralized or distilled water useful for drinking purposes? If not, how can it be made useful?
Answer: Although very pure, demineralized or distilled water is not useful for drinking purposes and this is because it does not contain even useful minerals. To make it useful for drinking purposes, useful minerals in proper amounts should be added to demineralized or distilled water.
Question 17. Describe the usefulness of water in biosphere and biological systems.
Answer:
Water is essential for the survival of various life forms on Earth. It comprises around 65-70% of the body mass of flora and fauna.
- Due to its elevated specific heat, thermal conductivity, surface tension, dipole moment, and dielectric constant relative to other liquids, water is essential to the biosphere.
- The significant heat of vaporization of water enables it to regulate body temperature. Water also contributes indirectly to climate regulation.
- Water facilitates the transfer of minerals, nutrients, and enzymes throughout the body and influences metabolic processes.
- Water is an essential element in the process of photosynthesis. Consequently, rivers play a crucial function in the ecosystem.
Question 18. Knowing the properties of H20 and D20, do you think that D20 can be used for drinking purposes?
Answer: Drinking heavy water (D2O) is harmful to the human beings. Heavy water being highly hygroscopic, absorbs water from different parts of the body. As a result, the body cells may get destroyed. Apart from this, heavy water slows down different biochemical reactions such as mitosis, cell division, etc.
Question 19. What is the difference between the terms ‘hydrolysis’ and ‘hydration’?
Answer: Hydrolysis refers to the reaction of salt with water to form acidic or basic solutions.
For example:
⇒ \(\mathrm{Na}_2 \mathrm{CO}_3+2 \mathrm{H}_2 \mathrm{O} \rightleftharpoons \underset{\text { (basic solution) }}{2\left[\mathrm{Na}^{+}+\mathrm{OH}^{-}\right]+\mathrm{H}_2 \mathrm{CO}_3 ;}\)
⇒ \(\mathrm{NH}_4 \mathrm{Cl}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \underset{\text { (acidic solution) }}{\left[\mathrm{H}^{+}+\mathrm{Cl}^{-}\right]+\mathrm{NH}_4 \mathrm{OH}}\)
Hydration, on the other hand, refers to the addition of H20 to ions or molecules to form hydrated ions or hydrated salts. For example:
⇒ \(\underset{\text { Salt }}{\mathrm{NaCl}(s)+\mathrm{H}_2 \mathrm{O}} \rightarrow \underbrace{\mathrm{Na}^{+}(a q)+\mathrm{Cl}^{-}(a q)}_{\text {Hydrated ions }}\)
⇒ \(\begin{array}{cc} \mathrm{CuSO}_4(s)+5 \mathrm{H}_2 \mathrm{O}(l) & \rightarrow \mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}(s) \\ \text { Anhydrous salt } & \text { Hydrated salt } \\ \text { (colourless) } & \text { (blue) } \end{array}\)
Question 20. How can saline hydrides remove traces of water from organic compounds?
Answer: Saline hydrides (i.e., NaH, CaH2, etc.) react with water forming their corresponding metal hydroxides with the liberation of dihydrogen. Thus, traces of water present in organic solvent can be easily removed by distilling them over saline hydrides when dihydrogen escapes into the atmosphere, metal hydroxides are left in the distillation flask, and the dry organic solvent is distilled over.
Class 11 Chemistry Chemical Properties Of Hydrogen
Question 21. What do you expect the nature of hydrides to be if formed by elements of atomic numbers 15, 19, 23, and 44 with dihydrogen? Compare their behavior towards water.
Answer:
The element with Z = 15 is non-metal phosphorus and hence it forms the covalent hydride PH3.
The element with Z = 19 is the alkali metal potassium and hence it forms the saline or ionic hydride K+HT.
The element with Z = 23 is the transition metal vanadium of group-3 and hence it forms the interstitial hydride (VHj 6).
The element with Z = 44 is the transition metal ruthenium (Ru) belonging to group 8. It does not form anhydride (hydride gap). Only the ionic hydride, KH reacts with water evolving dihydrogen.
⇒ \(\mathrm{KH}(s)+\mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{KOH}(a q)+\mathrm{H}_2(g)\)
Question 22. Do you expect different products in solution when aluminum (III) chloride and potassium chloride are treated separately with
Normal water
Acidified water and
Alkaline water? Write equations wherever necessary.
Answer: In normal water: KC1, being a salt of a strong acid and strong base, does not undergo hydrolysis in normal water. It simply dissociates to form K+(aq) and Cl~(aq) ions.
⇒ \(\mathrm{KCl}(s) \stackrel{\text { water }}{\longrightarrow} \mathrm{K}^{+}(a q)+\mathrm{Cl}^{-}(a q)\)
On the other hand, A1C13, being a salt of a weak base Al(OH)3 and a strong acid HC1, undergoes hydrolysis giving acidic solution.
⇒ \(\mathrm{AlCl}_3(s)+3 \mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{Al}(\mathrm{OH})_3(s)+3 \mathrm{H}^{+}(a q)+3 \mathrm{Cl}^{-}(a q)\)
In acidified water: The H+ ions react with Al(OH)3 to form Al3+(aq) ions and H20. Therefore, in acidic water, A1C13 exists as A13+(aq) and Cl~(aq) ions.
⇒ \(\mathrm{AlCl}_3(s) \stackrel{\text { acidified } \mathrm{H}_2 \mathrm{O}}{\longrightarrow} \mathrm{Al}^{3+}(a q)+3 \mathrm{Cl}^{-}(a q)\)
⇒ \(\mathrm{KCl}(s) \stackrel{\text { acidified water }}{\longrightarrow} \mathrm{K}^{+}(a q)+\mathrm{Cl}^{-}(a q)\)
In alkaline water: A1(0H)3 reacts with OH ions to form soluble tetrahydroxoaluminate complex or meta-aluminateion (A10ÿ2).
KC1 does not react and dissociate to give K+(aq) and Cl~(aq) ions.
MPBSE Class 11 Chemistry Chemical Properties Of Hydrogen
Question 1. What is ‘hydrogenite’? Mention its use.
Answer: A mixture of silicon, caustic soda, and slaked lime is called hydrogenite. Dihydrogen can be prepared by heating hydrogenate with water.
⇒ \(\mathrm{Si}+2 \mathrm{NaOH}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Na}_2 \mathrm{SiO}_3+\mathrm{H}_2 \uparrow\)
∴ \(\mathrm{Si}+\mathrm{Ca}(\mathrm{OH})_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{CaSiO}_3+2 \mathrm{H}_2 \uparrow\)
Question 2. What is denoted by [Hg04]+ ion? Explain
Answer: High charge density and high hydration energy of the H+ ion (proton) cause the H+ ion to remain solvated to form a hydroxonium ion or hydronium ion (H30+). Hydronium ion again forms hydrogen bonds with three water molecules and remains solvated. Therefore, in water, a proton forms [H3O(H2O)3]+ or [H9O4]+ ion.
Question 3. Pure para-hydrogen is available but not pure ortho hydrogen. Explain.
Answer: At ordinary temperature, ordinary hydrogen is a mixture of 75% of ortho and 25% of para-isomer. With a decrease in temperature, the amount of ortho-hydrogen decreases while that of para-hydrogen increases. At 20K, pure para-hydrogen is obtained. As para-hydrogen is more stable, it is found in the pure form. However, if the temperature is increased, the amount of ortho-isomer of dihydrogen increases but at 400K or above, the ratio of ortho-and para-isomer is fixed (3: 1). Therefore, pure para-hydrogen is available but not pure ortho-hydrogen.
Question 4. How many hydrogen-bonded water molecule(s) are associated with CuS04-5H20?
Answer: Only one molecule of water, which remains outside the brackets (coordination sphere) is linked by a hydrogen bond to SO as shown below. The remaining four water molecules are associated to Cu2+ ion by coordination bonds.
Question 5. Write down the reaction between H2OZ and hydrazine (NH2NH2) in the presence of Cu(II) catalyst. Mention the use of this reaction.
Answer: A highly concentrated solution (about 40%) of H202 (called high test peroxide) oxidizes hydrazine (N2H4) in the presence of Cu(II) into nitrogen gas, itself being oxidized to water (steam).
⇒ \(\stackrel{-2}{\mathrm{~N}_2} \mathrm{H}_4(l)+2 \mathrm{H}_2{ }_{-1}^{-1}(l) \underset{\text { catalyst }}{\stackrel{\mathrm{Cu}(\mathrm{II})}{\longrightarrow}} \stackrel{0}{\mathrm{~N}}(\mathrm{~g})+4 \mathrm{H}_2 \stackrel{-2}{\mathrm{O}}(g)+\text { heat }\)
The reaction is highly exothermic and is accompanied by a large increase in volume which in turn generates high pressure. Due to this, the reaction is employed for propelling rockets.
MPBSE Class 11 Chemistry Chemical Properties Of Hydrogen
Hydrogen Multiple Choice Questions And Answers
Question 1. The normality of’30 volume’ of H2O2 is—
- 2.678 (N)
- 5.336 (N)
- 8.034 (N)
- 6.685 (N)
Answer: 2. 5.336 (N)
Volume strength = 5.6 x normality
or, 30= 5.6 x normality
⇒ \(\text { or, normality }=\frac{30}{5.6} \mathrm{~N}=5.357 \mathrm{~N}\)
The normality of 30 volumes of H202 is 5.357N.
Question 2. A commercial sample of H202 is labeled as 10V. Its % strength is nearly
- 3
- 6
- 9
- 12
Answer: 1. 3
10V (10 volume) H202 means that lmL of H202 solution will produce lOmL of 02 at STP. Now, the decomposition of H2O2 is as below:
⇒ \(\begin{array}{lr} 2 \mathrm{H}_2 \mathrm{O}_2 \rightarrow & 2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2 \\ (2 \times 34) \mathrm{g} & 22400 \mathrm{~mL} \\ =68 \mathrm{~g} & \text { (at STP) } \end{array}\)
At STP, lOmL of 02 is obtained from lmL of H202
⇒ \(22400 \mathrm{~mL} \text { of } \mathrm{O}_2 \text { is obtained from } \frac{22400}{10} \mathrm{~mL} \text { of } \mathrm{H}_2 \mathrm{O}_2\)
= 2240 mL of H202
2240mL of H202 solution contains 68g of H202
∴ \(100 \mathrm{~mL} \text { of } \mathrm{H}_2 \mathrm{O}_2 \text { solution contains } \frac{68 \times 100}{2240}=3.03 \mathrm{~g}\) of H202 = 3g of H202
The percent strength of 10V H2O2 is 3
Class 11 Chemistry Chemical Properties Of Hydrogen Question 3. In aqueous alkaline solution, two-electron reduction of HO2 gives
- HO
- H2O
- 02
- O2–
Answer: 1. Ho
Question 4. Which statement is not correct for ortho- and para-hydrogen
- They have different boiling points
- Ortho-form is more stable than para-form
- They differ in their nuclear spin
- The ratio of ortho to para-hydrogen changes with a change in temperature
Answer: 2. Ortho-form is more stable than para-form
At normal or high temperatures, ortho-hydrogen is more stable than para-hydrogen but at very low temperatures para-hydrogen is more stable than orthohydrogen.
Question 5. At room temperature, the reaction between water and fluorine produces—
- HF and H202
- HF,02 and F202
- F©, 02 and H®
- HOF and HF
Answer: 3. F©, 02 and H®
Question 6. Which one of the following statements about water is false
- Water can act both as an acid and as a base
- There is extensive intramolecular hydrogen bonding in the condensed phase
- Ice formed by heavy water sinks in normal water
- Water is oxidized to oxygen during photosynthesis
Answer: 2. There is extensive intramolecular hydrogen bonding in the condensed phase
Water molecules are associated with the formation of intermolecular hydrogen bonding.
Question 7. Hydrogen peroxide oxidises [Fe(CN)6]4– to [Fe(CN)6]3– in acidic medium but reduces [Fe(CN)6]3~ to [Fe(CN)6]4– in alkaline medium. The other products formed are, respectively
- H20 and (H20 + 02)
- H20 and (H20 + OH”)
- (H20 + 02) and H20
- (H20 + 02) and (H20 + OH-)
Answer: 1. H20 and (H20 + 02)
Acidic medium:
Question 8. Which of the following statements about hydrogen is incorrect
- Hydrogen has three isotopes of which tritium is the most common
- Hydrogen never acts as a cation in ionic salts
- Hydronium ion, H30 exists freely in solution
- Dihydrogen does not act as a reducing agent
Answer: 4. Dihydrogen does not act as a reducing agent
Among three isotopes of hydrogenprotlum (H1), deuterium H2), and tritium (H3), normal hydrogen is protium. Among these three, tritium is radioactive and unstable for which it is negligible in amount. In an aqueous solution, metal is reduced by dihydrogen. Again, metallic oxide is also reduced by dihydrogen to free metal.
Question 9. In ice, the oxygen atom is surrounded
- Tetrahedrallyby 4 hydrogen atoms
- Octahedrallyby 2 oxygen and 4hydrogen atoms
- Tetrahedrallyby 2 hydrogen 2 oxygen atoms
- Octahedrallyby 6 hydrogen atoms.
Answer: 1. X-ray studies have shown that in ice, four hydrogen atoms tetrahedrally surround each oxygen atom.
Class 11 Chemistry Chemical Properties Of Hydrogen MCQs Question 10. Predict the product of the reaction of I2 with H202 in a basic medium.
- I-
- I2O3
- I03
- I3
Answer: 1. I-
Question 11. Strength of H202 is 15.18 g.L-1 , then it is equal to—
- 1 volume
- 10 volume
- 5 volume
- 7 volume
Answer: 3. 5 Volume
⇒ \(\text { Volume strength }=\frac{5.6 \times \text { strength in } \mathrm{g} \cdot \mathrm{L}^{-1}}{\text { equivalent mass of } \mathrm{H}_2 \mathrm{O}_2}\)
∴ \(=\frac{5.6 \times 15.18}{17}=5 \text { volumes }\)
Question 12. Which of the following reactions increases the production of dihydrogen from synthesis gas—
- \(\mathrm{CH}_4(g)+\mathrm{H}_2 \mathrm{O}(g)\stackrel{\mathrm{1270K}}{\mathrm{Ni}} \mathrm{CO}(g)+\mathrm{H}_2(g)\)
- \(\mathrm{C}(g)+\mathrm{H}_2 \mathrm{O}(g) \stackrel{1270 \mathrm{~K}}{\longrightarrow} \mathrm{CO}(g)+\mathrm{H}_2(g)\)
- \(\mathrm{CO}(g)+\mathrm{H}_2 \mathrm{O}(g) \underset{\text { Catalyst }}{\stackrel{673\mathrm{~K}}{\longrightarrow}}\mathrm{CO}_{2(g)}+\mathrm{H}_{2(g)}\)
- \(\mathrm{C}_2 \mathrm{H}_6(g)+2 \mathrm{H}_2 \mathrm{O}(g) \underset{\mathrm{Ni}}{\stackrel{1270 \mathrm{~K}}{\longrightarrow}} 2 \mathrm{CO}+5 \mathrm{H}_2\)
Answer: 3. \(\mathrm{CO}(g)+\mathrm{H}_2 \mathrm{O}(g) \underset{\text { Catalyst }}{\stackrel{673\mathrm{~K}}{\longrightarrow}}\mathrm{CO}_{2(g)}+\mathrm{H}_{2(g)}\)
The production of dihydrogen can be increased by reacting carbon monoxide of syngas with steam in the presence ofiron chromate as catalyst.
⇒ \(\mathrm{CO}_{(\mathrm{g})}+\mathrm{H}_2\mathrm{O}_{(\mathrm{g})} \underset{\text { Catalyst}}{\stackrel{673\mathrm{~K}}{\longrightarrow}}\mathrm{CO}_{2(\mathrm{~g})}+\mathrm{H}_{2(\mathrm{~g})}\)
Question 13. Which of the following reactions produces hydrogen
- Mg + H20
- H2S2O8 + H2O
- Ba02 + HCl
- Na202 + 2HC1
Answer: 1. Mg + H20
Alkali and alkaline earth metals react with water to produce hydrogen gas and metal hydroxides. This occurs due to high electropositive character ofthe metals.
⇒ \(\mathrm{Mg}+2 \mathrm{H}_2 \mathrm{O} \longrightarrow\mathrm{Mg}(\mathrm{OH})_2+\mathrm{H}_2\)
Question 14. H202 can be obtained when following reacts with H2S04 except with
- Ba02
- Pb02
- Na202
- SrOz
Answer: 2. Pb02
H202 is prepared by the reaction of peroxide with H2S04.Pb02 is a dioxide. Hence, it does not give H202 with dilute H2S04.
MPBSE Class 11 Chemistry Chemical Properties Of Hydrogen
Very Short-Type Questions
Question 1. Which is the lightest gas known?
Answer: Dihydrogen
Question 2. Which isotope of hydrogen is radioactive?
Answer: Tritium (jH)
Question 3. Give examples of an ionic, a covalent, and a metallic hydride.
Answer: CaH2 (ionic); NH3 (covalent) & CrH (metallic)
Question 4. What is hydrolith?
Answer: Calcium hydride (CaH2)
Chemical Properties Of Hydrogen
Question 5. Name the two nuclear spin isomers of dihydrogen.
Answer: ortho- hydrogen and
Question 6. Give an example of an electron-deficient hydride in which three centre-two electron bonds are present.
Answer: para- hydrogen; 6. B2Hg
Question 7. Which gaseous compound on treatment with dihydrogen produces methanol?
Answer: Carbon monoxide;
Question 8. How will you prove that a colorless liquid is water?
Answer: 1. White anhydrous CuS04 becomes blue in contact with water
Question 9. What is the unit for expressing the degree of hardness of water?
Answer: ppm (parts per million);
Question 10. Write the names of two chemical substances that are used for removing dissolved oxygen from water meant for the boiler.
Answer: Hydrazine (NH2NH2) and sodium sulphite (Na2S03);
Question 11. Why is heavy water used in atomic reactors?
Answer: It is used as a moderator
Question 12. Name a solid and a liquid absorbent of water.
Answer: Cone. H2S04 and P205;
Question 13. Which chemical is commercially known as ‘perhydrol’?
Answer: H202 solution;
Question 14. What is called ‘hyper lol or horizon’?
Answer: 2-ethylanthraquinol;
Chemical Properties Of Hydrogen Question 15. What is the die volume strength of a molar solution of H202?
Answer: A compound of hydrogen peroxide and urea is called hyper lol (NH2C0NH2-H202);
Question 16. Which organic reagent is used for the manufacture of H2O2?
Answer: 2-ethylanthraquinol;
Question 17. 10 volume of H202 = x(N)H202 .What is the value of x?
Answer: 17. X = 56; 18.
Question 18. What are how water molecules are bonded to the anhydrous salt to form hydrates?
Answer: Coordinate bond and H bond
MPBSE Class 11 Chemistry Chemical Properties Of Hydrogen Short-Type Questions
Question 1. How can dihydrogen be obtained from nitric add?
Answer: By the reaction of Mg with HN03(2%)
Question 2. Concentrated H2S04 cannot be used for drying H2 gas why?
Answer: Cone. H2S04 on absorbing H20 from moist H2 produces so much heat that hydrogen catches fire
Chemical Properties Of Hydrogen Question 3. What type of hydrides can be formed by each of the following elements: Li, Zr, P, Hf, N, Ca?
Answer: Ionic, metallic, covalent, metallic, covalent, and ionic respectively.
Question 4. What are the different types of bonds formed by hydrogen in its compounds?
Answer: Ionic, covalent and H-bond
Question 5. In the preparation of H202, MnOz or Pb02 cannot be used instead of Ba02 —why?
Answer: Ba02 contains peroxo linkage ( —O —O — ), but Mn02(0=Mn=0) or Pb02(0=Pb=0) does not contain peroxo linkage.
MPBSE Class 11 Chemistry Chemical Properties Of Hydrogen Numerical Problems
Question 1. 1L of a hard water sample contains 1 mg CaCl2 and 1 mg MgCl2. Estimate the degree of hardness of this sample of water.
Answer: Molecular mass of CaCl2 =111
Nowlllg of CaCl2= lOOg of CaC03
⇒ \(1 \mathrm{mg} \mathrm{CaCl}_2 \equiv \frac{100 \times 0.001}{111} \mathrm{~g}^2 \text { of } \mathrm{CaCO}_3\)
= 9 X 10-4g of CaC02 = 0.9mg of CaC03
The molecular mass of MgCl2 = 95
Now, 95g of MgCl2=100g of CaC03
⇒ \(1 \mathrm{mg} \text { of } \mathrm{MgCl}_2 \equiv \frac{100}{95} \mathrm{mg} \text { of } \mathrm{CaCO}_3=1.05 \mathrm{mg} \text { of }\)CaC03
Equivalent amount of CaC03 corresponding to CaCl2
and MgCl2 presentin or 103g of hard water
= (0.90 + 1.05)mg = 1.95mg [1L water = 103g = 106mg water]
The mass of the equivalent amount of CaC03 corresponding to CaCl2 and MgCl2 present in 106mg of water is 1.95 mg. Hence, the degree of hardness of the given sample is 1.95 ppm.
Hence, the hardness of that sample of water is 75 ppm.
Chemical Properties Of Hydrogen
Question 2. Determine the strength of ’30 volume’ H202 in normality.
Answer: Volume strength = 5.6 x normality
or, 30 = 5.6 x normality
⇒ \(\text { or, normality }=\frac{30}{5.6} \mathrm{~N}=5.35 \mathrm{~N}\)
The normality of 30 volume of H202 is 5.35N
Question 3. Determine the volume (in liter) of 02 obtained at STP when 0.1 liter of 2(M)H2O2 solution is decomposed.
Answer:
Now, 1L1(M) 1I202 = 34g of H202
1L2(M) H202 → (2 X 34)g of H202
0.1L 2(M) H202 = (2 X 34 X 0.1 )g of H202 = 6.8g of H2O2
Again at STP, 68g of M202 produces 22.4L of 02.
⇒ \(6.8 \mathrm{~g} \text { of } \mathrm{H}_2 \mathrm{O}_2 \text { produces } \frac{22.4}{68} \times 6.8 \mathrm{~L} \text { of } \mathrm{O}_2\)
= 21241, of 02
Question 4. When 100 ml of tube-well water is titrated using methyl orange as an indicator, it requires 15 ml of 0.01 (N) HCl. Estimate the hardness of that sample of water.
Answer: 15 mL 0.01 (N) HCI = 1 mL 0.15(N) HCI
⇒ \(1000 \mathrm{~mL} 1(\mathrm{~N}) \mathrm{HCl} \equiv \frac{100}{2} \mathrm{~g} \mathrm{CaCO}_3\)
⇒ \(1 \mathrm{~mL} 0.15(\mathrm{~N}) \mathrm{HCl}=50 \times \frac{1}{1000} \times 0.15 \mathrm{~g} \text { of } \mathrm{CaCO}_3\)
= 7.5x 10-3g of CaCO3
Therefore, 100ml, or 100g of that sample of water contains some hardness-producing substance which is equivalent to 7.5 x 10-3g of CaC03. 106g of water contains the hardness producing substance equivalent to\(\frac{7.5 \times 10^{-3} \times 10^6}{100}=75 \mathrm{~g} \text { of } \mathrm{CaCO}_3\)
Chemical Properties Of Hydrogen
Question 5. Calculate the amount of H202 present in 600 mL of 10-volume H202 solution.
Answer: 10 volume H202 means that 1 mL of H2O2 solution will produce 10 mL of 02 at STP
⇒ \(\begin{gathered} 2 \mathrm{H}_2 \mathrm{O}_2 \longrightarrow 2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2 \\ \left(2^{\prime} 34\right) \mathrm{g}=68 \mathrm{~g} \quad 22400 \mathrm{~mL} \text { (at STP) } \end{gathered}\)
At STP, 10 mL 02 is obtained from 1 mL of lOvol H202 solution.
⇒ \(22400 \mathrm{~mL} \text { of } \mathrm{O}_2 \text { is obtained from } \frac{22400}{10} \mathrm{~mL} \text { of } 10 \mathrm{vol}\)
H202 solution =2240 mL of H202
2240 mL of H202 solution contains 68g of H202
⇒ \(100 \mathrm{~mL} \text { of } \mathrm{H}_2 \mathrm{O}_2 \text { solution contains } \frac{68 \times 100}{2240}=3.03 \mathrm{~g} \text { of }\)H2O2.
⇒ \(\text { So, } 600 \mathrm{~mL} \text { of } \mathrm{H}_2 \mathrm{O}_2 \text { solution contains }=\frac{3.03 \times 600}{100} \mathrm{~g} \text { of }\)
H202 = 18.18g of H202 =18.2g of H202
Chemical Properties Of Hydrogen
Question 6. An excess of acidic KI solution is added to 25 mL of a H202 solution when iodine is liberated. 20 mL of 0.1(N) sodium thio-sulfate solution is required to titrate the liberated iodine. Calculate the percentage strength, volume strength, and strength in normality of the H202 solution.
Answer: 25 x ;e(N) = 20 x 0.1(N)
⇒ \(x=\frac{20\times0.1}{25}=\frac{2}{25}=0.08(\mathrm{~N})\)
Amount of H202 in 25mL0.08(N) II202 solution
1000 mL 1(N) H202 solution = 17g H202
⇒ \(\begin{aligned} & 25 \mathrm{~mL} 0.08(\mathrm{~N}) \mathrm{H}_2 \mathrm{O}_2 \text { solution } \equiv \frac{17 \times 25 \times 0.08}{1000}=0.034 \mathrm{~g} \\ & \mathrm{H}_2 \mathrm{O}_2 \end{aligned}\)
⇒ \(100 \mathrm{ml} 0.08(\mathrm{~N}) \mathrm{H}_2 \mathrm{O}_2 \text { solution contains }=\frac{0.034 \times 100}{25}\)
= 0.136g H2O2
% strength of H202 solution = 0.136
68g H202 gives → 22.4 L 02 at STP
⇒ \(0.034 \mathrm{~g} \mathrm{H}_2 \mathrm{O}_2 \text { solution } \rightarrow \frac{22.4 \times 0.034}{68} \text { Litre } \mathrm{O}_2 \text { at STP }\)
= 0.0112 Litre 02 at STP
= 11.2 mL 02 at STP
25 mL H2O2 solution gives 11.2 mL 02 at STP.
⇒ \(\text { ImL } \mathrm{H}_2 \mathrm{O}_2 \text { solution gives } \frac{11.2}{25}=0.448 \mathrm{~mL} \mathrm{O}_2 \text { at STP. }\)
Volume strength of the given H202 solution = 0.448