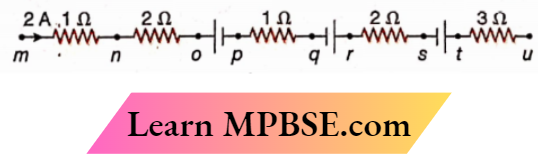Class 12 Physics Ohm’s Law Multiple Choice Question And Answers
Question 1. Two copper wires have a ratio of 1: 4 between their diameters. If the same current passes through both of them, the drift velocity of the electrons will be in the ratio of
- 16:1
- 4: 1
- 1:4
- 1:16
Answer: 1. 16:1
Question 2. A conductor of uniform cross-section is carrying a current of 1 ampere. The number of free electrons flowing across the cross-section of the conductor per second is
- 6.25 x 1018
- 6.25 x 1017
- 6.25 X 1016
- 6.025 x 1023
Answer: 1. 6.25 x 1018
1 A electric current = the flow of 1 C charge through the cross-sectional area of the conductor in 1 second.
∴ Number of free electrons flowing per second
⇒ \(\frac{1 \mathrm{C}}{\text { charge of an electron }}\)
= \(\frac{1 \mathrm{C}}{1.6 \times 10^{-19} \mathrm{C}}\)
= \(6.25 \times 10^{18}\)
The option 1 is correct.
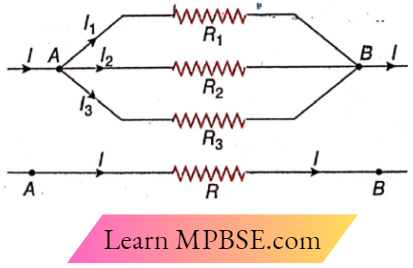
Question 3. In the circuit,
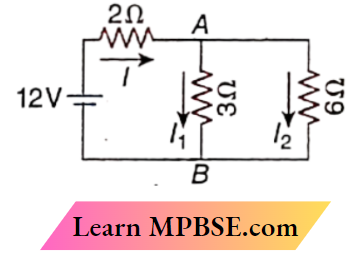
- I = 3A
- I1 = 2A
- I2 = 1A
- VAB = 8V
Answer:
1. I = 3A
2. I1 = 2A
3. I2 = 1A
Question 4. In the circuit,
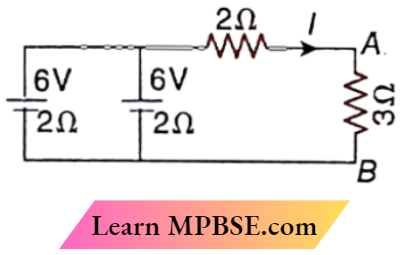
- I = 1 A
- I = \(\frac{4}{3}\)A
- VAB = 4 V
- VAB = 3 V
Answer:
1. I = 1 A
4. VAB = 3 V
Class 12 Physics Ohm’s Law MCQs Question 5. If I = 2 A in the circuit
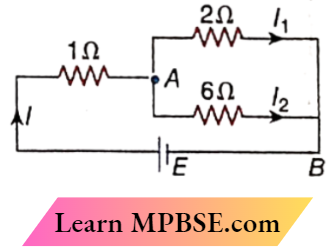
- E = 5 V
- I1 = 1.5 A
- I2 = 0.5 A
- VAB = 3 V
Answer:
1. E = 5 V
2. I1 = 1.5 A
3. I2 = 0.5 A
4. VAB = 3 V
Question 6. If the resistance of the rheostat Rh is gradually increased in the circuit,
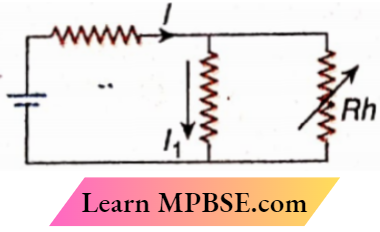
- I will rise gradually
- I will fall gradually
- I1 will rise gradually
- I1 will fall gradually
Answer:
2. I will fall gradually
3. I1 will rise gradually
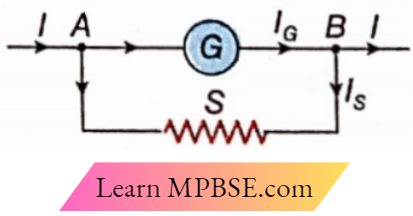
Question 7. Which of the following observations is correct if the galvanometer resistance is 200Ω in the circuit
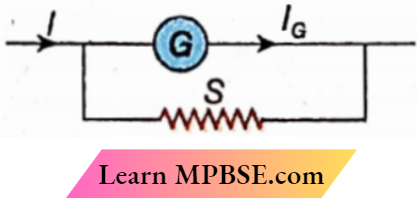
- S = 5Ω, I = 1.5 A,IG = 36.6 mA
- S = 1Ω, I = 1.5 A, IG = 14.9 mA
- S = 2Ω, I = 2 A, IG = 19.8 mA
- S = 3Ω, I = 2 A, IG = 29.6 mA
Answer:
1. S = 5Ω, I = 1.5 A,IG = 36.6 mA
3. S = 2Ω, I = 2 A, IG = 19.8 mA
4. S = 3Ω, I = 2 A, IG = 29.6 mA
Question 8. Brown, black, orange, and gold are the respective colors of the characteristic rings on a carbon resistor. Which of the following values of its resistance are definitely wrong?
- 10.6 kΩ
- 10.2 kΩ
- 9.8 kΩ
- 9.4 kΩ
Answer:
1. 10.6 kΩ
4. 9.4 kΩ
Question 9. Three 4Ω resistances can be connected in different combinations. The probable values of the equivalent resistance are
- 12Ω
- 6Ω
- \(\frac{10}{3}\)Ω
- \(\frac{4}{3}\)Ω
Answer:
1. 12Ω
2. 6Ω
4. \(\frac{4}{3}\)Ω
Class 12 Physics Ohm’s Law MCQs Question 10. E1, E2, and r1,r2 are respectively, the emf’s and internal resistances of two cells. The current through an external resistance R, when it is connected to the first cell, is equal to that when it is connected to the second. Here, the probable relations are
- E1 = E2,r1 = r2
- E1 > E2, r1 > r2
- E1 < E2, r1 < r2
- E1 > E2, r1 < r2
Answer:
1. E1 = E2,r1 = r2
2. E1 > E2, r1 > r2
3. E1 < E2,r1 < r2
Question 11. A voltmeter and an ammeter are connected in series to an ideal cell of emf E. The voltmeter reading is V and the ammeter reading is I. Choose the correct options.
- The voltmeter resistance is \(\frac{V}{I}\)
- The potential difference across the ammeter is (E- V)
- V<E
- Voltmeter resistance + ammeter resistance = \(\frac{E}{I}\)
Answer:
1. The voltmeter resistance is \(\frac{V}{I}\)
2. The potential difference across the ammeter is (E- V)
3. V
Question 12. Part of a circuit. Which points have the potential same as that of point m?
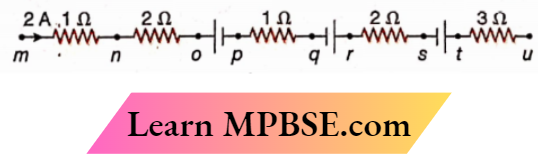
- p
- r
- t
- u
Answer:
2. r
3. t
Question 13. A galvanometer has a resistance of 100Ω and a full-scale range of 50μA. It can be used as a voltmeter or as a higher range ammeter, provided a resistance is added to it. Pick the correct range and resistance combination(s).
- 50 V range with 10kΩ resistance in series
- 10 V range with 200 kΩ .resistance in series
- 5 mA range with 1Ω resistance in parallel
- 10 mA range with 1Ω resistance in parallel
Answer:
2. 10 V range with 200 kΩ .resistance in series
3. 5 mA range with 1Ω resistance in parallel
Question 14. A straight conductor AB lies along the axis of a hollow metal cylinder, which is connected to the earth through a conductor C. A quantity of charge will flow through C if
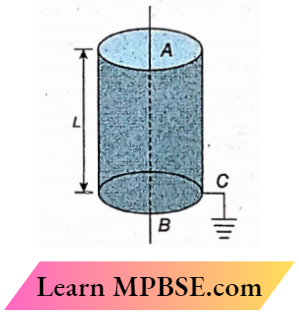
- A Current begins to flow through AB
- The current through AB is reversed
- AB is removed and a beam of protons flows in its place
- AB is removed, and a beam of electrons flows in its place
Answer:
3. AB is removed and a beam of protons flows in its place
4. AB is removed, and a beam of electrons flows in its place
Class 12 Physics Ohm’s Law MCQs Question 15. To double the full-scale voltage reading of any galvanometer turned into a voltmeter, you must
- Increase the resistance to 3R
- Half the resistance R
- Increase the resistance to 4R
- None of the above
Answer: 4. None of the above
Question 16. The resistance Ω of a conducting wire depends on its material, length l, and area of cross-section A. The resistivity of the material of the wire is p = \(\frac{RA}{l}\) the value of p is different for different materials. It is very low for conducting materials, like metals. Besides, the resistance of a conductor also depends on its temperature. If the resistance of a conductor is R0 at 0° C, and Rt at t° C, then Rt = R0(1 + αt), where a is called the temperature coefficient of resistance. The resistance increases with temperature for metallic conductors but decreases for graphite, a few metal alloys, and for semiconductors like silicon and germanium.
1. The resistance of a metal wire increases by 10% when its temperature rises from 10° C to 110° C. The temperature coefficient of resistance of the metal is
- 0.02 °C-1
- 0.01 °C-1
- 0.002 °C-1
- 0.001 °C-1
Answer: 4. 0.001 °C-1
2. The length of this metal wire is doubled by stretching. What will be the change in its resistance?
- 100% increase
- 200% increase
- 300% increase
- 500% decrease
Answer: 3. 300% increase
3. The temperature of this new wire is again raised from A B 10°C to 110°C. The percentage increase of its resistance would be
- 5%
- 10%
- 20%
- 40%
Answer: 2. 10%
4. The temperature coefficient of resistance of a semiconductor is
- Zero
- Positive
- Negative
- Positive or negative depending on the material
Answer: 3. Negative
5. The graphs of the relations between current (I) and potential difference (V) of a metal wire at two different temperatures t1 and t2. The relation between t1 and t2 is
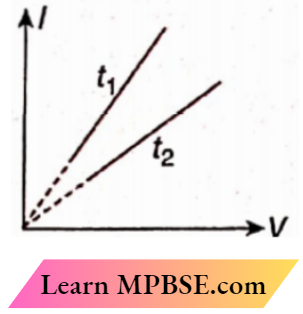
- t1 = t2
- t1 < t2
- t1 > t2
- Insufficient data
Answer: 2. t1 < t2
Question 17. If a current passes through a metal conducting wire of area of cross-section A, the drift velocity of free electrons inside the metal is vd = \(\frac{1}{neA}\) where the amount of electric charge of an electron = e, and the number of free electrons per unit volume of the metal =n. The applied electric field on the wire is E = \(\frac{V}{l}\) y, where a potential difference V exists between two points,l apart, along the length of the wire. If R is the resistance of the wire between those two points, then the resistivity of its material is \(\rho=\frac{R A}{l}\). Besides, the mobility (μ) of the free electrons inside a wire is defined as their drift velocity for a unit-applied electric field.
1. Two copper wires have both lengths and radii in the ratio 1: 2. If the ratio between the electric currents flowing through them is also 1: 2, what would be the ratio between the drift velocities of free electrons?
- 1:1
- 1:2
- 2:1
- 4:1
Answer: 3. 2:1
2. The radii of two wires of the same metal are in the ratio 1: 2. The same potential difference is applied between two points at a distance on each of the wires. The ratio between the drift velocities of the free electrons in two wires is
- 1:1
- 1:2
- 2:1
- 1:4
Answer: 2. 1:2
Class 12 Physics Ohm’s Law MCQs 3. The radii of two wires, made of two different metals, are in the ratio 1: 2. The number density of free electrons in the first metal is double that in the second metal. If the current in the first wire is 1 A, then the current In the second wire producing the same drift velocity is
- 1 A
- 2 A
- 4 A
- 8 A
Answer: 3. 4 A
4. Tire current through unit cross-section of a conductor, culled tire electric current density J, Is related to the applied electric field E as
- J = \(\rho\)E
- \(J=\frac{1}{\rho} E\)
- J = μE
- \(J=\frac{1}{\mu} E\)
Answer: 2. \(J=\frac{1}{\rho} E\)
Question 18. Measurements and Interpretations of voltage and electric current signals are common In modern medicine. Occasionally, a situation arises In which a voltmeter or an ammeter Is needed but it Is not available. A galvanometer Is an instrument dial that can be used to construct an ammeter (for measuring electric currents). It can also be used to construct a voltmeter (to measure voltages). In both cases, a resistor R must be connected to the galvanometer to effect the change. To turn the galvanometer into an ammeter, the resistor R is connected in parallel. The resistor R is connected in series with the galvanometer in order to turn it into a voltmeter. The current required to produce a full-scale deflection in a galvanometer is 10mA. The internal resistance of the galvanometer is 100Ω. Let Vr be the voltage across r and VR the voltage across R.
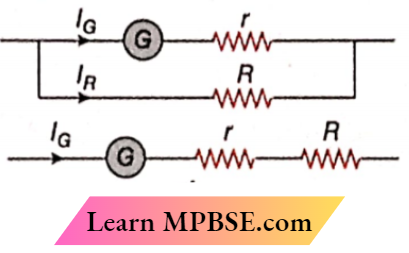
1. Which of the following relations correctly applies to the ammeter?
- Vr>VR
- Vr < VR
- Vr=VR
- More information is required
Answer: 1. Vr>VR
2. What resistance must be connected in parallel to the galvanometer to turn It into an ammeter capable of reading electric currents up to 10.01 A?
- 0.1Ω
- 1Ω
- 10Ω
- None
Answer: 1. 0.1Ω
3. What resistance R must be connected in series to the galvanometer in order to convert it to a 100 V voltmeter?
- 900Ω
- 1000Ω
- 9900Ω
- 10000Ω
Answer: 3. 9900Ω
4. In the voltmeter circuit, the current in the resistor R must be
- Negligible, so that it has only a small effect on the voltage reading
- Substantial, but does not have any effect on the voltage reading
- Substantial, but does have some effect on the voltage reading
- None of the above
Answer: 1. Negligible, so that it has only a small effect on the voltage reading
Class 12 Physics Ohm’s Law MCQs 5. Which of the following relations correctly applies to the voltmeter circuit?
- Vr>VR
- Vr<VR
- Vr=VR
- Vr = 2VR
Answer: 2. Vr<VR
Question 19. Two cells each of emf e but internal resistances r1 and r2 are connected in series through an external resistance R. If the potential difference across the first cell is zero while current flows, the relation of R in terms of r1 and r2 is
- R = r1 + r2
- R = r1-r2
- R = \(\frac{1}{2}\) (rj + r2)
- R = \(\frac{1}{2}\)(r1 – r2)
Answer: 2. R = r1-r2
Question 20. Resistance of the thinner wire is 10Ω, then the resistance of the other wire will be
- 40Ω
- 20Ω
- 10Ω
- 5Ω
Answer: 3. 10Ω
⇒ \(R=\rho \frac{l}{A}=\frac{\rho l}{\pi r^2}\)
∴ \(\frac{R_1}{R_2}=\frac{\rho_1}{\rho_2} \cdot \frac{l_1}{l_2} \cdot\left(\frac{r_2}{r_1}\right)^2=\frac{1}{3} \times \frac{1}{3} \times\left(\frac{3}{1}\right)^2=1\)
R2 = R1 = 10Ω
The option 3 is correct
Question 21. Four cells, each of emf E . and internal resistance r, are connected in series across an external resistance R. By mistake one of the cells is connected in reverse. Then the current in the external circuit is
- \(\frac{2 E}{4 r+R}\)
- \(\frac{3 E}{4 r+R}\)
- \(\frac{3 E}{3 r+R}\)
- \(\frac{2 E}{3 r+R}\)
Answer: 1. \(\frac{2 E}{4 r+R}\)
Currently the circuit,
⇒ \(I=\frac{3 E-E}{4 r+R}=\frac{2 E}{4 r+R}\)
The option 1 is correct.
Class 12 Physics Ohm’s Law MCQs Question 22. A circuit consists of three batteries of emf E1 = 1V, E2 = 2 V, and E3 = 3V and internal resistances 1Ω, 2Ω, and 1Ω respectively which are connected in parallel. The potential difference between points P and Q is
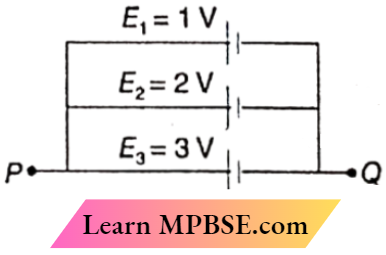
- 1.0V
- 2.0V
- 2.2V
- 3V
Answer: 2. 2.0V
Equivalent resistance of the internal resistances connected in parallel
= \(\frac{2}{5}\)Ω
Total current = \(\frac{1}{1}+\frac{2}{2}+\frac{3}{1}\)
= 5V
∴ The potential difference between points P and
Q = 5 x \(\frac{2}{5}\)
= 2V
The option 2 is correct
Question 23. A metal wire of a circular cross-section has a resistance. The wire is now stretched without breaking so that its length is doubled and the density is assumed to remain the same. If the resistance of the wire now becomes R2 then R2: R1 is
- 1:1
- 1:2
- 4:1
- 1:4
Answer: 3. 4: 1
If the length of the wife is l and its cross-sectional area is A, the volume of the wire, V = lA = constant.
Then, A = \(\frac{V}{l}\)
Now, from the relation \(R=\rho \frac{l}{A}\)
⇒ \(\frac{R_1}{R_2}=\frac{l_1}{l_2} \cdot \frac{A_2}{A_1}=\frac{l_1}{l_2} \cdot \frac{V / l_2}{V / l_1}=\left(\frac{l_1}{l_2}\right)^2=\left(\frac{1}{2}\right)^2=\frac{1}{4}\)
or, \(\frac{R_2}{R_1}=\frac{4}{1}=4: 1\)
The option 3 is correct
Question 24. Two equal resistances, 400Ω each, are connected in series with an 8 V battery. If the resistance of the first one increases by 0.5%, the charge required in the resistance of the second one in order to keep the potential difference across it unaltered is to
- Increase it by 1Ω
- Increase it by 2Ω
- Increase it by 4Ω
- Decrease it by 4Ω
Answer: 2. Increase it by 2Ω
Increase in first resistance =400 x \(\frac{0.5}{100}\) = 2Ω
Initially, the emf 8 V will be divided equally between the two resistances. So the voltage across each resistance will be 4 V. When the first resistance is increased, the second resistance should also be increased by 2Ω to keep the voltage across it unchanged.
The option 2 is correct.
Class 12 Physics Ohm’s Law MCQs Question 25. Two wires of the same radius having lengths l1 and l2 and resistivities p1 and p2 are connected in series. The equivalent resistivity will be
- \(\frac{\rho_1 l_2+\rho_2 l_1}{\rho_1+\rho_2}\)
- \(\frac{\rho_1 l_1+\rho_2 l_2}{l_1+l_2}\)
- \(\frac{\rho_1 l_1-\rho_2 l_2}{l_1-l_2}\)
- \(\frac{\rho_1 l_2+\rho_2 l_1}{l_1+l_2}\)
Answer: 2. \(\frac{\rho_1 l_1+\rho_2 l_2}{l_1+l_2}\)
⇒ \(\frac{\rho_1 l_1}{A}+\frac{\rho_2 l_2}{A}=\rho_{\mathrm{eq}} \frac{\left(l_1+l_2\right)}{A}\)
∴ \(\rho_{\mathrm{eq}}=\frac{\rho_1 l_1+\rho_2 l_2}{l_1+l_2}\)
The option 2 is correct.
Question 26. The effective resistance between A and B is \(\frac{7}{12}\)Ω if each side of the cube has 1Ω resistance. The effective resistance between the same two points when the link AB is removed is
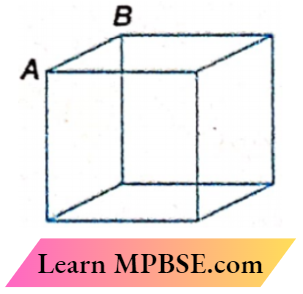
- \(\frac{7}{12}\)Ω
- \(\frac{5}{12}\)Ω
- \(\frac{7}{5}\)Ω
- \(\frac{5}{7}\)Ω
Answer: 3. \(\frac{7}{5}\)Ω
If x is the effective resistance between A and B of the remaining cube, then
⇒ \(\frac{7}{12}=\frac{1 \times x}{1+x}\)
Solving we get, x = \(\frac{7}{5}\)Ω
The option 3 is correct
Question 27. Four resistors 100Ω, 200Ω, 300Ω, and 400Ω are connected to form four sides of a square. The resistors can be connected in any; order. What is the maximum possible equivalent resistance across the diagonal of the square?
- 210Ω
- 240Ω
- 300Ω
- 250Ω
Answer: 4. 250Ω
The resistance across the diagonal of the square formed by the four resistors is equal to the equivalent resistance of the resistors in parallel combination.
The equivalent resistance of the parallel combination is maximum when the resistance on the two sides of the diagonal is equal.
∴ Equivalent resistance,
⇒ \(R=\frac{500 \times 500}{500+500}=250 \Omega\)
The option 4 is correct.
Class 12 Physics Ohm’s Law MCQs Question 28. What will be the current through the 200Ω resistor in the given circuit a long time after the switch K is made on?
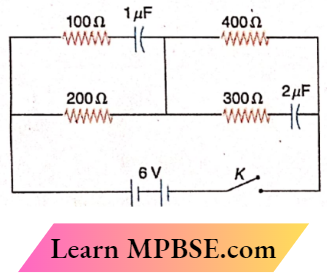
- 0
- 100mA
- 10mA
- 1mA
Answer: 3. 10mA
A long time after the switch K is turned on, the 1μF and 2μF capacitors will be open-circuited and no current will flow through them.
∴ Current through, the 200Ω resistor
⇒ \(\frac{6}{200+400}=\frac{1}{100} \mathrm{~A}\)
= 10mA
The option 3 is correct.
Question 29. When the 5 V potential difference is applied across a wire of length 0.1 m, the drift speed of electrons is 2.5 x 10-4 m s-1. If the electron density in the wire is 8 x 1028 m-3, the resistivity of the material is close to
- 1.6 x 10-8Ω.m
- 1.6 x 10-7Ω.m
- 1.6 x 10-6Ω.m
- 1.6 x 10-5Ω.m
Answer: 4. 1.6 x 10-5Ω.m
Drift velocity, \(v=\frac{I}{n e A}\)
⇒ \(I=\frac{V}{R}=\frac{V}{\rho \frac{l}{A}} \quad \text { or, } \rho=\frac{V}{\frac{I}{A} l}=\frac{V}{n e v l}\)
∴ \(\rho=\frac{5}{\left(8 \times 10^{28}\right) \times\left(1.6 \times 10^{-19}\right) \times\left(2.5 \times 10^{-4}\right) \times 0.1}\)
= 1.56 X 10-5Ω.m
= 1.6 X 10-5Ω m
The option 4 is correct.
Question 30. The temperature dependence of resistances of Cu and undoped Si in the temperature range of 300-400 K, is best described by
- Linear increase for Cu, linear increase for Si
- Linear increase for Cu, exponential increase for Si
- Linear increase for Cu, exponential decrease for Si
- Linear decrease for Cu, linear decrease for Si
Answer: 3. Linear increase for Cu, exponential increase for Si
Question In the given circuit the current in each resistance is
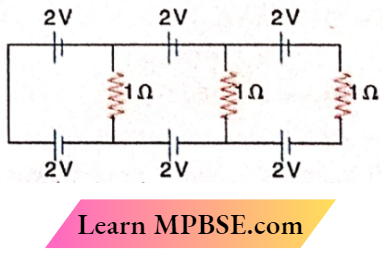
- 1A
- 0.25A
- 0.5A
- zero
Answer: 4. zero
There are two cells of equal electromotive force in opposite directions with each other in each loop. So, the electromotive force in each loop is zero. Hence, the current is also zero.
Option 4 is correct
Class 12 Physics Ohm’s Law MCQs Question 31. In the given circuit diagram when the current reaches a steady state in the circuit, the charge on the capacitor of capacitance C will be
- CE
- \(\frac{C E r_1}{r_2+r}\)
- \(\frac{C E r_2}{r+r_2}\)
- \(\frac{C E r_1}{r_1+r}\)
Answer: 3. \(\frac{C E r_2}{r+r_2}\)
The current reaches a steady state in the circuit means that the current through the capacitor is zero
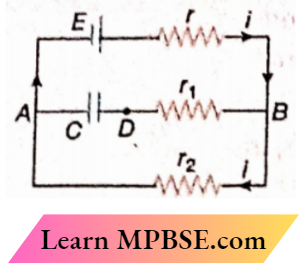
∴ Current in the circuit, \(i=\frac{E}{r+r_2}\)
Potential differences across the capacitor,
⇒ \(V_{A D}=V_{A B}=i r_2=\frac{E r_2}{r+r_2}\)
Therefore, the charge storedin the capacitor C,
⇒ \(Q=C V_{A D}=C E \frac{r_2}{r+r_2}\)
The option 3 is correct
Question 32. A, B, and C are voltmeters of resistance R, 1.5R, and 3R respectively. When some potential difference is applied between X and Y, the voltmeter readings are VA, VB, and VC respectively. Then
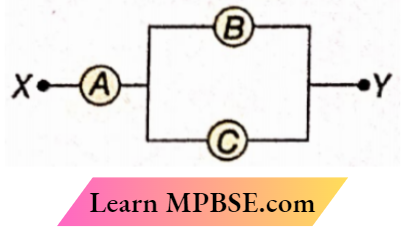
- VA=VB=VC
- VA ≠ VB = VC
- VA = VB ≠ VC
- VA ≠ VB ≠ VC
Answer: 1. VA=VB=VC
Clearly, VB = VC
Again, equivalent resistance for B and C = \(\frac{1.5 \times 3}{1.5+3}=1 \Omega\)
= resistance of A.
Hence, VA = VB = VC
The option 1 is correct.
Question 33. Across a metallic conductor of a non-uniform cross-section, a constant potential difference is applied. The quantity which remains constant along the conductor is
- Current density
- Current
- Drift velocity
- Electric field
Current remains constant along the conductor.
The option 2 is correct.
Class 12 Physics Ohm’s Law MCQs Question 34. In the electrical circuit, the current I through the side AB is
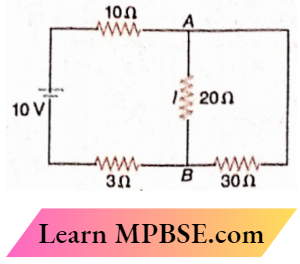
- \(\frac{6}{2}\)A
- \(\frac{10}{33}\)A
- \(\frac{1}{5}\)A
- \(\frac{10}{63}\)A
Answer: 1. \(\frac{6}{2}\)A
E=IR
or, \(10=I\left(10+\frac{20 \times 30}{20+30}+3\right)=0.25 I\)
Here, \(I=\frac{10}{25}=\frac{2}{5} \mathrm{~A}\)
∴ \(I_{A B}=\frac{2}{5} \times \frac{30}{20+30}=\frac{6}{25} \mathrm{~A}\)
The option 1 is correct
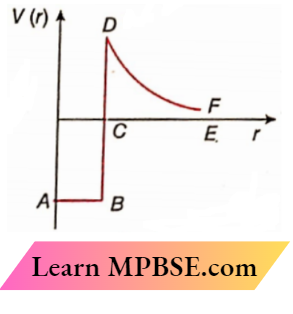
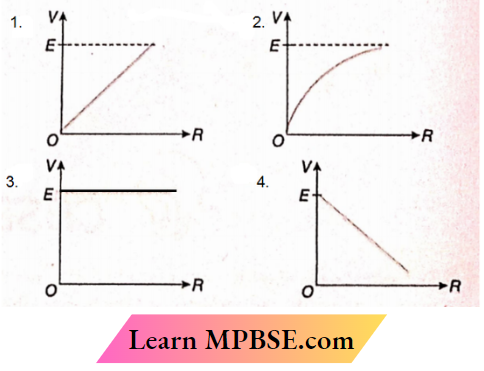
Question 35. A cell of emf E and internal resistance r is connected to a variable external resistor R. The graph which gives the terminal voltage of cell V with respect to R is
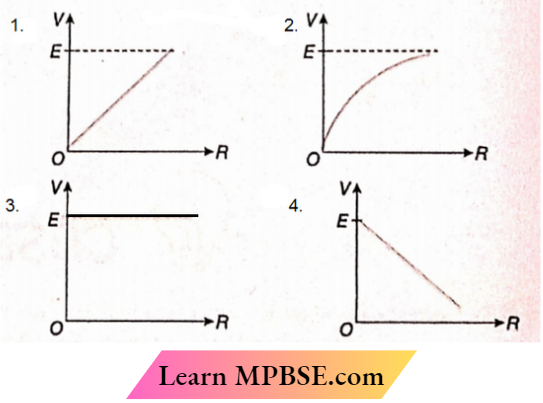
Answer: 2.
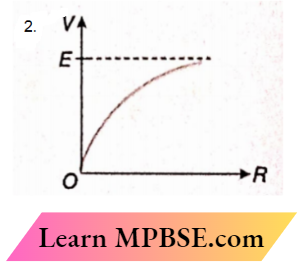
⇒ \(V=E-I r=E-\frac{E r}{R+r}\)
or, \(\frac{d V}{d R}=\frac{E r}{(R+r)^2}\)
Therefore, the slope of the V-R graph is positive and it decreases within resistance R
The option 2 is correct.
Question 36. A carbon resistor of (47 ± 4.7)kΩ is to be marked with rings of different colors for its identification. The color code sequence will be
- Yellow—Green—Violet—Gold
- Yellow—Violet—Orange—Silver
- Violet—Yellow—Orange—Silver
- Green—Orange—Violet—Gold
Answer: 2. Yellow—Violet—Orange—Silver
⇒ \((47 \pm 4.7)=47 \pm\left(\frac{4.7}{47} \times 100\right) \%=47 \pm 10 \%\)
∴ \((47 \pm 4.7) \mathrm{k} \Omega=47 \times 10^3 \pm 10 \% \Omega\)
Therefore, the color code sequence will be yellow—Violet—Orange—Silver
The option 2 is correct
Class 12 Physics Ohm’s Law MCQs Question 37. A set of n equal resistors, of value R each, are connected in series to a battery of emf E and internal resistance R. The current drawn is I. Now, the n resistors are connected in parallel to the same battery. Then the current drawn from the battery becomes 10I. The value of n is
- 20
- 11
- 10
- 9
Answer: 3. 10
Equivalent resistance in series,
R1 = nR + R = (n + 1)R
∴ \(I=\frac{E}{R_1}=\frac{E}{(n+1) R}\)…..(1)
Equivalent resistance in parallel
⇒ \(R_2=\left(\frac{R}{n}+R\right)=\left(1+\frac{1}{n}\right) R\)
∴ \(10 I=\frac{E}{R_2}=\frac{E}{\left(1+\frac{1}{n}\right) R}\)…..(2)
From equations (1) and (2)
⇒ \(\frac{10 I}{I}=\frac{(n+1) R}{\left(1+\frac{1}{n}\right) R} \text { or, } 10\left(\frac{n+1}{n}\right)=(n+1)\)
or, n = 10
The option 3 is correct.
Question 38. A battery consists of a variable number n of identical cells (having internal resistance r each) which are connected in series. The terminals of the battery are short-circuited and the current I is measured. Which of the graphs shows the correct relationship between I and n?
Answer: 3.
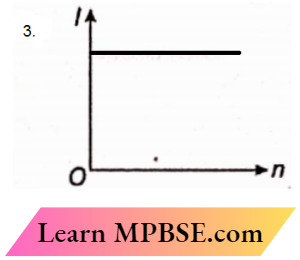
Current in the circuit containing n identical cells connected in series,
I = \(\frac{ne}{nr}\) [ e = emf of each identical cell]
or, I = \(\frac{e}{r}\)
So, I remain the same with any change of n.
The option is correct.