Class 11 Chemistry
MPBSE Class 11 Chemistry Hydrogen Multiple Choice Question and Answers
MPBSE Class 11 Chemistry Hydrogen Multiple Choice Question and Answers
Question 1. At absolute zero
- Only para-hydrogen exists
- Onlypara- hydrogen exists
- Both ortho- and para-hydrogen exist
- Neither para- nor ortho-hydrogen exists
Answer: 1. Only para-hydrogen exists
Question 2. In which of the following reaction dihydrogen acts as an oxidising agent—
- F2 + H2 → 2HF
- Cl2 + H2 → 2HC1
- N2 + 3H2 →2NH3
- 2Na + H2 → 2NaH
Answer: 4. 2Na + H2 → 2NaH
Question 3. Which of the following halogens has the least affinity towards hydrogen—
- I2
- CI2
- Br2
- F2
Answer: 1. I2
Question 4. Which of the following compounds on electrolysis produces hydrogen—
- dil. H2S04
- dil. solution of NaOH
- Ba(OH)2 solution
- KOH solution
Answer: 3. Ba(OH)2 solution
MPBSE Class 11 Chemistry MCQs Question 5. The thermal stability of Gr.-15 hydrides follows the order
- ASH3 > PH3 > NH3 > SbH3 > BiH3
- NH3 > PH3 > ASH3 > SbH3 > BiH3
- NH3 > AsH3 > PH3 > SbH3 > BiH3
- BiH3 > SbH3 > AsH3 > PH3 > NH3
Answer: NH3 > PH3 > ASH3 > SbH3 > BiH3
Question 6. The correct order of vaporization enthalpy of the following hydride is
- NH3<PH3<AsH3
- AsH3<PH3<NH3
- PH3<AsH3<NH3
- NH3<AsH3<PH3
Answer: 3. PH3<AsH3<NH3
Question 7. Interstitial hydrides are formed by—
- S-block elements
- P-block elements
- D-block elements
- Intert gas elements
Answer: 3. D-block elements
Question 8. The correct descending order of thermal stability of alkali metals hydrides is—
- LiH > NaH > KH > RbH > CsH
- CsH > RbH > KH > NaH > LiH
- NaH > KH > LiH > CsH > RbH
- CsH > LiH > KH > NaH > RbH
Answer: 1. LiH > NaH > KH > RbH > CsH
MPBSE Class 11 Chemistry MCQs Question 9. Solubility of NaClin the solvents H20 and DaO is
- Equal in both
- More in D20
- More in H20
- Only in H20
Answer: 3. More in H20
Question 10. The degree of hardness of 1L sample water containing 0.002 mol MgS04 is
- 20 ppm
- 200 ppm
- 2000ppm
- 120ppm
Answer: 2. 200 ppm
Question 11. Which of the following reacts with water to produce electron-precise hydrides—
- Ca3P2
- AI4C3
- Mg3N2
- None of these
Answer: 2. Al4C3
Hydrogen Class 11 MCQ Question 12. Which of the following couples reacts with water to produce the same gaseous product—
- K and K02
- K and K02
- Na and Na202
- Ba and Ba02
Answer: 2. K and K02
Question 13. Which of the following compounds contain free hydrogen
- Water
- Marsh gas
- Water gas
- Acid
Answer: 3. Water gas
Question 14. Which of the following reacts with metallic sodium to produce hydrogen—
- CH4
- C2H6
- C2H4
- C2H2
Answer: 4. C2H2
Question 15. Semi-water gas is
- CO + H2 + N2
- H2 + CH4
- CO + H2+ O2
- CO + H2
Answer: 1. CO + H2 + N2
Question 16. Which of the following metals does not react with cold water liberates H2 with boiling water
- Na
- K
- Pt
- Fe
Answer: 4. Fe
Question 17. Volume of ’10 volume’ H202 required to convert 0.01 mol PbS into PbS04 is—
- 11.2 mL
- 22.4 mL
- 33.6 mL
- 44.8 mL
Answer: 4. 44.8 mL
Hydrogen Class 11 MCQ Question 18. On dilution of H202, the value of dielectric constant
- Increases
- Remains same
- Decreases
- None of these
Answer: 1. Increases
Question 19. By which of the following water gets oxidized to oxygen
- Cl02
- KMn04
- H202
- F2
Answer: 4. F2
Question 20. Which of the following does not get oxidized by H202
- Na2S03
- PBS
- KI
- O3
Answer: 4. O3
Question 21. The temperature at which the density of D20 is maximum is
- 9°C
- 11.5°C
- 15.9°C
- 20°C
Answer: 11.5°C
Question 22. Which of the following undergoes disproportionation reaction with water—
- S03
- F2
- Cl2
- N2
Answer: 2. F2
Hydrogen Class 11 MCQ Question 23. Which of the following undergoes disproportionation reaction with water—
- S03
- F2
- Cl2
- N2
Answer: 3. Cl2
Question 24. The non-inflammable hydrides
- NH3
- PH3
- ASH3
- SbH3
Answer: 1. NH3
Question 25. The triple point of water is
- 203K
- 193K
- 273K
- 373K
Answer: 3. 273K
Question 26. The process by which hydrogen is prepared by the reaction of silicon, iron alloy, and NaOH is
- Woodprocess
- Haber’s process
- Silicol process
- Bosch process
Answer: 3. Silicol process
Question 27. An element reacts with hydrogen to form a compound A, which in reaction with water liberates hydrogen again. The elements—
- Cl
- CS
- Se
- N2
Answer: 2. CS
Question 28. Only one element of which of the following groups forms a metal hydride
- Gr-6
- Gr-7
- Gr-8
- Gr-9
Answer: 1. Gr-6
Question 29. An acidic solution of which of the following turns orange in the presence of H202 —
- Ba02
- Na202
- Ti02
- Pb02
Answer: 3. Ti02
Question 30. In the following reaction the isotopic oxygens \(2 \mathrm{MnO}_4^{-}+3 \mathrm{H}_2 \mathrm{O}_2^{18} \rightarrow 2 \mathrm{MnO}_2+3 \mathrm{O}_2+2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{OH}^{-}\)
- Both get converted into 02
- Both get converted into oh
- Both get converted into more
- One of them gets converted to 02, another to mn02
Answer: Both get converted into 02
Question 31. X on electrolysis produces Y which on vacuum distillation produces H202. The numbers of peroxo linkage present X and Y are
- 1,1
- 1,2
- 0>1
- 0,0
Answer: 3. 0>1
Question 32. The compound which on electrolysis in its molten or liquid state liberates hydrogen at the anode is
- NaOH
- CaH2
- HC1
- H20
Answer: 2. CaH2
Question 33. Which of the following couples exhibit the maximum isotope effect
- H D
- O O
- CI CI
- C C
Answer: 1. H D
Question 34. Which of the following emits by tritium
- Neutron
- Ray
- Particle
- Particle
Answer: 3. Particle
MPBSE Class 11 Chemistry MCQs Question 35. Oxidation of benzene by H202 in the presence of ferrous sulfate produces
- Pheno
- Cyclohexane
- Anisole
- Benzaldehyde
Answer: 1. Pheno
Question 36. The oxidation state of Cr in the product obtained by the reduction of K2Cr20? by atomic hydrogen is
- +6
- +2
- 0
- +3
Answer: 4. +3
Question 37. Which of the following does not get reduced by H2 in its aqueous solution
- Cu2+
- Fe3+
- Zn2+
- Ag+
Answer: 3. Zn2+
Question 38. Which of the following compounds has a similar odor as that of H202
- Caustic soda
- Chloroform
- Alcohol
- Nitric acid
Answer: 4. Nitric acid
Question 39. Which of the following compounds reacts with atomic hydrogen to form formaldehyde
- CO
- C02
- CH4
- C2H2
Answer: 1. CO
Question 40. Which of the following isotopes of hydrogen is the most reactive
- H
- H
- H
- All the isotopes are equally reactive
Answer: 1. H
MPBSE Class 11 Chemistry MCQs Question 41. When equal amounts of Zn are allowed to react separately with excess H2S04 and excess NaOH, then the ratio of the volumes of hydrogen produced for the first and the second case respectively
- 1:2
- 2:1
- 4:9
- 1:1
Answer: 4. 1:1
Question 42. Which of the following hydrides of s-block elements have a polymeric structure
- LiH
- BeH2
- No
- MgH2
Answer: 2. BeH2
Question 43. Which of the following statements is true—
- Ifz= 15, the element forms a covalent hydride
- Ifz= 23, the element forms an ionic hydride
- Ifz= 19, the element forms an ionic hydride
- Ifz= 44, the element forms metalic hydride
Answer: 1. Ifz= 15, the element forms a covalent hydride
Question 44. Which of the following hydrides are polynuclear hydrides
- No
- C3H8
- N2H4
- HF
Answer: C3H8
Question 45. Which of the following statements is correct
- Metallic hydrides are hydrogen-deficient
- Metallic hydrides are conductors of heat and electricity
- Ionic hydrides in their solid state do not conduct electricity
- Ionic hydrides on electrolysis in their molten state produce h2 at the cathode.
Answer: 1. Metalic hydrides are hydrogen deficient
Question 46. Which of the following ions get exchanged with Na+ ion of zeolite when zeolite is added to thehard water
- H+ ion
- Ca2+ ion
- So+ ion
- Mg2+ ion
Answer: 2. Ca2+ ion
MPBSE Class 11 Chemistry MCQs Question 47. Which of the following reactions are neurolysis
- 2Na + 2D20 → 2NaOD + D2
- AlClg + 3D20 →Al(OD)3 + 3DC1
- Ca + 2D20→ Ca(OD)2 + D2
- Fe2(S4)3 + 6D20-> 2Fe(0D)3 + 3D2S04
Answer: 2. AlClg + 3D20→ Al(OD)3 + 3DC1
Question 48. Which of the following reactions are redox reactions
- H2O + so2 → H2SO3
- CaO + H20 → Ca(OH)2
- 2Na + 2H20 → 2NaOH + H2
- 2F2 + 2H20 → 02 + 4HF
Answer: 3. 2Na + 2H20 → 2NaOH + H2
Question 49. In which of the following reactions H202 acts as a reductant—
- CgHg + H202 → CgHgOH + H220
- PbS + 4H202 → PbS04 + 4H20
- Na0Br +H202 → NaBr + H20 + 02
- 2Mn04 +6H+ + 5H202 → 2Mn2+ + 8H20 + 502
Answer: 3. Na0Br +H202 → NaBr + H20 + 02
Question 50. Which of the properties are the same for a metal and its hydride
- Hardness
- Electrical conductance
- Magnetic property
- Metallic lustre
Answer: 1. Hardness
Question 51. The correct orders are—
- H2 < D2 < T2 : boilingpoint
- H2 < D2 < T2 : freezing point
- H2 < D2 < T2 : latent heat ofvaporisation
- T2O > H20 > D20 : dissociation constant
Answer: 1. H2 < D2 < T2 : boilingpoint
Question 52. Which of the following reacts with zinc to produce hydrogen gas
- dil.HCl
- cold water
- hot NaOH solution
- cone. H2S04
Answer: 2. cold water
Question 53. Which of the following properties has a greater magnitude in D20 than that in H20
- Viscosity
- Surface tension
- dielectric constant
- latent heat of vaporisation
Answer: 2. Viscosity
Question 54. Which of the following metal hydrides get reduced by hydrogen
- CuO
- Pb304
- Na202
- MgO
Answer: 1. CuO
Question 55. Multimolecular covalent hydrides of s-block are
- LiH
- BeH2
- NaH
- MgH2
Answer: 2. BeH2
MPBSE Class 11 Chemistry MCQs Question 56. The oxidation numbers of the most electronegative element in the product were obtained due to the reaction between Ba02 and dil. H2S04 are—
- -1
- 0
- -2
- +1
Answer: 1. -1
Question 57. Which of the following compounds decreases the rate of decomposition of H202 —
- CO(NH2)2
- Mn02
- PbNHCOCH3
- (COOH)2
Answer: 1. CO(NH2)2
Question 58. Which of the following produces H202 on hydrolysis
- Pemitricacid
- Perchloric acid
- Perdisulphuric acid
- Caro’s acid
Answer: 1. Pemitricacid
Question 59. Choose the correct statements
- The concentration of 20 volume H202 solution is 60.7g L-1
- volume strength of 2(N)H202 solution is 15
- volume strength of 2(N)H202 solutions 11.2
- The concentration of the 20-volume H202 solution is 50.7g. L-1
Answer: 1. The Concentration of 20-volume H202 solution is 60.7g L-1
Question 60. Choose the correct alternative—
- a mixture of HCl and HCIO is formed when chlorine reacts with cold water
- arrange color of K2Cr207 solution turns blue when it reacts with H202
- under low pressure, isopropyl alcohol reacts with a small amount of H202 to produce formaldehyde
- hydrolith produces black coloured product when it reacts with PbS04
Answer: 1. a mixture of HC1 and HCIO is formed when chlorine reacts with cold water
Question 60. Which of the following alternatives is not true—
- The correct order of reactivity of h2 towards the halogens
- Is: cl2 > br2 > i2 > f2
- The concentration of h202 used in rockets is 90%
- H2 gets more readily absorbed on the surface of metal than d2
- Conversion of atomic hydrogen into molecular hydrogens is an exothermic process
Answer: 1. Correct order of reactivity of h2 towards the halogens
MPBSE Class 11 Chemistry Notes For Entropy
MPBSE Class 11 Chemistry Notes For Concept Of Entropy
We have already seen that enthalpy is not the ultimate criterion of spontaneity. Another factor such as the randomness of the constituting particles (molecules, atoms, or ions) of the system may also be responsible for determining the spontaneity of a process.
Rudolf Clausius introduced a new thermodynamic property or state function known as entropy, from the Greek word ‘trope’ meaning transformation. It is denoted by the letter ‘S’. The entropy of a system is a measure of the randomness or disorderliness of its constituent particles.
The more disordered or random state of a system, the higher the entropy it has. Thus, from the molecular point of view, the entropy of a system can be defined below.
Class 11 Chemistry Notes For Entropy
The entropy of the system is a thermodynamic property that measures the randomness or disorderliness of the constituent particles making up the system.
According to the above definition, it may seem that entropy is related to the individual constituent particle of the system. However, thermodynamics, whose framework is based on a macroscopic approach, is not concerned with the existence and the nature of the constituent particles of the system.
Entropy, which is a macroscopic property, is in no way related to the behavior of the individual atoms or molecules of a system, instead, it reflects the average behavior of a large collection of atoms or molecules by which a system usually consists.
MPBSE Class 11 Chemistry Notes For Mathematical interpretation of entropy
Since heat (q) is not a state function, the exchange of heat (5q) i.e., the amount of heat absorbed or rejected by a system during a process is not an exact differential.
But in a reversible process, the ratio of the heat exchanged between the system and surroundings \(\frac{\delta q_{r e v}}{T}\) to an absolute temperature at which the heat exchanger takes place is an exact differential. Hence, the quantity indicates the change ofa state function. This function is called entropy (S). Therefore, the change in entropy,
⇒ \(d S=\frac{\delta q_{r e v}}{T}=\frac{\begin{array}{c}
\text { Reversible heat transfer between } \\
\text { system and its surroundings }
\end{array}}{\begin{array}{c}
\text { Temperature (K) at which } \\
\text { heat is transferred }
\end{array}}\) …………………………….(1)
a reversible process, the state of a system changes from state 1 (initial state) to state 2 (final state), then the change in entropy (AS) in the process can be determined by integrating the equation
⇒ \(\int_1^2 d S=\int_1^2 \frac{\delta q_{r e v}}{T} \text { or, } S_2-S_1=\int_1^2 \frac{\delta q_{r e v}}{T} \text { or, } \Delta S=\int_1^2 \frac{\delta \tilde{q} q_{r e t}}{T}\)
For the process occurring at a constant temperature, the change in entropy, \([\Delta S=\frac{1}{T} \int_1^2 \delta q_{r e v}=\frac{q_{r e v}}{T}.\)
Class 11 Chemistry Notes For Entropy
It is not possible to define entropy; however, we can define the change in entropy of a system ( dS or AS) undergoing a reversible process. It is defined as the ratio of reversible heat exchange between the system and its surroundings to the temperature at which the heat exchange takes place.
The relation tells us for a given input of heat into a system, the entropy of the system increases more at a lower temperature than at a higher temperature.
The randomness in a system is a measure of its entropy. The more randomness the more entropy. Therefore, for a given input of heat into a system, the randomness of the system increases more when heat is added to the system at a lower temperature than at a higher temperature.
MPBSE Class 11 Chemistry Notes For Characteristics of entropy
The entropy of a system quantifies the unpredictability of its constituent particles. Entropy is a state function as its value for a system relies solely on the current state of the system, and its change (ΔS) during a process is determined exclusively by the beginning and final states of the system, independent of the pathway taken to execute the process.
- Being a state function, it is a quantity that is independent of the path taken. Entropy is an extended property, as its value for a system is contingent upon the quantity of matter within the system.
- The entropy of the cosmos grows in a spontaneous process (ΔSuniv > 0) and decreases in a non-spontaneous process (ΔSuniv < 0). At equilibrium, the change in entropy of the system is zero. At absolute zero, the entropy of a pure, perfect crystalline solid is zero.
MPBSE Class 11 Chemistry Notes For Physical significance of entropy
There exists a relationship between the entropy of the system and the randomness of its constituent particles (atoms, ions, or molecules).
The entropy of a system increases or decreases with the increase or decrease in randomness of the particles constituting the system. Therefore, entropy is the measure of the randomness of the constituent particles in a system. this is the physical significance of entropy.
Class 11 Chemistry Notes For Entropy
The change in entropy is defined in terms of a reversible process, for which it is defined as
⇒ \(d S=\frac{\delta q_{r e v}}{T}\) where 8qrev) Is the reversible exchange of heat between a system and its surroundings at 7’K. in case of an irreversible process, the change in entropy
⇒ \(d S \neq \frac{\delta q_{i r r}}{T}\) where 5<](rr represents irreversible exchange of heat between a system and its surroundings at 7’K.
As entropy is a state function, its change in a particular process does not depend on the nature of the process. Thus, the change in entropy in a process carried out reversibly is the same as the change in entropy that occurs if the same process is carried out irreversibly.
MPBSE Class 11 Chemistry Notes For Unit of Entropy
In the CGS system, the unit of entropy = cal.deg-1, while the unit of entropy in SI = J. K-1
Change In Entropy Of The System In Some Processes
When a system undergoes a process, its change in entropy in the process is ΔS = S2 – S1; where S1 and S2 are the entropies of the initial and final states of the system respectively, in a process.
In a process, if S2 > S1, then ΔS is positive. This means that the entropy of a system increases in the process. For example, the melting of ice or vaporization of water is associated with an increase in the entropy of the system, so AS is involved in these processes.
If S2 < S1, then ΔS is negative, indicating that the entropy of the system decreases in the process. For example, when ice is formed from liquid water or water is formed from water vapor, the entropy of the system decreases i.e., ΔS =-ve.
MPBSE Class 11 Chemistry Notes For Change in entropy in a chemical reaction
In any chemical reaction, the initial entropy (S1) of the system means the total entropy of the reactants, and the final entropy of the system (S2) means the total entropy of the products.
Hence, the change in entropy in a chemical reaction, ΔS = \(S_2-S_1^{3 i}=\sum S_{\text {products }}-\sum S_{\text {reactants }} \text {, where, } \sum S_{\text {reactants }}\text { and } \sum S_{\text {products }}\)
Change in entropy of the system in a cyclic process:
The change in entropy of the system in any process, ΔS = S2– S1 where S1 and S2 are the initial and final entropies of the system, respectively. Because entropy is a state function, and in a cyclic process the initial and the final states of a system are the same, = S2, and the change in entropy ofthe system, ΔS = 0.
MPBSE Class 11 Chemistry Notes For Change in entropy of the system in a reversible adiabatic process
In an adiabatic process, heat exchange does not occur between a system and its surroundings. Therefore, in a reversible adiabatic process, qrev = 0 and the change in entropy ofthe system in this process.
⇒ \(d S=\frac{\delta q_{r e v}}{T}=\frac{0}{T}=\mathbf{0} \text { or, } d s=0 \text { or, } \Delta S=0\)
Therefore, the change in entropy of a system undergoing a reversible adiabatic process is zero, i.e., the entropy of a system remains the same in an adiabatic reversible change. Owing to this a reversible adiabatic process is sometimes called an isentropic process.
Class 11 Chemistry Notes For Entropy
Change in entropy of the system in an irreversible adiabatic process: Like reversible adiabatic process, heat exchange does not also occur in an irreversible adiabatic process. However, it can be shown that in an irreversible adiabatic process, the entropy change of a system is always positive, i.e., ΔS > 0.
MPBSE Class 11 Chemistry Notes For Change in entropy of the system in an isothermal reversible process
Let us consider, a system change from state 1 to state 2 in an isothermal reversible process. Therefore, in this process, the change in entropy of the system.
⇒ \(\int_1^2 d S=\int_1^2 \frac{\delta q_{r e v}}{T} \text { or, } S_2-S_1=\frac{1}{T} \int_1^2 \delta q_{r e v} \text { or, } \Delta S=\frac{q_{r e v}}{T}\)
Since T= constant as the process is isothermal]
MPBSE Class 11 Chemistry Notes For Change in entropy during a phase transition
Melting of a solid, vaporization of a liquid, solidification of a liquid, condensation of vapor, etc. are some examples of phase transition.
At a particular temperature, a phase transition occurs at a constant pressure. The temperature remains unaltered during the transition although heat is exchanged between the system and the surroundings.
Class 11 Chemistry Notes For Entropy
The phase transition can be considered as a reversible process. If qrev of heat is absorbed during a phase transition at constant pressure and 7’K, then the change in entropy, of the system. As the process is occurring at constant pressure
⇒ \(q_{\text {re }}=q_p=\Delta H.\).
hence \(\Delta s=\frac{\Delta n}{r}\) \(\Delta s=\frac{\Delta n}{r}\) ……………………….(1)
MPBSE Class 11 Chemistry Notes For The entropy of fusion
It In defined as the change in entropy associated with the transformation of one mole of a solid substance into its liquid phase at its melting point
According to equation (1) \(\Delta S_{f u s}=\frac{\Delta H_{f u s}}{T_f}\)
where ΔHvap= the enthalpy of fusion = die heat required for the transformation of 1 mol of a solid at its melting point into 1 ml of liquid and T1= melting point (K) of the given solid As the fusion of a solid substance is an endothermic process (Le, A> 0 ), the change in entropy due to fusion (ΔSvap) is always positive.
Class 11 Chemistry Notes For Entropy
In the solid phase of a substance, the constituent particles are held in an ordered state. The degree of orderliness is less in the liquid phase as the particles in the liquid have freedom of motion. This is why, when a solid melts, the randomness within the system increases, causing an increase in the entropy of the system.
Example: The enthalpy of fusion of ice at 0°C and 1 atm. Therefore, the change in entropy during the transformation of 1 mol of ice into 1 mol of water at 0°C and 1 atm is-
MPBSE Class 11 Chemistry Notes For Entropy of vaporization
It is defined as the change in entropy when one mole of a liquid at its boiling point changes to its vapor phase.
Where ΔH = the enthalpy of vaporization = the heat required for the transformation of 1 mol of liquid at its boiling point into 1 mol of vapor and Tb = boiling point ofthe liquid (K).
As the vaporization of a liquid is an endothermic process [i.e., \(\Delta H_{v a p}>0\)), the change in entropy in a vaporization process (ΔSvap) is always positive. When a liquid vaporizes, the molecular randomness in the system increases as the molecules in the vapor phase have more freedom of motion thus they have in the liquid phase. As a result, the vaporization of a liquid always leads increase in the entropy ofthe system.
Example: The enthalpy of vaporization of water at 100°C and 1 atm (ΔHvap) = 40.4 kj .mol-1
. Thus, the change in entropy due to the transformation of 1 mol of water into 1 mol of water vapor at 0°C temperature and 1 atm pressure is
⇒ \(\Delta S_{\text {vap }}=\frac{\Delta H_{\text {vap }}}{T_b}=\frac{40.4 \mathrm{~kJ} \cdot \mathrm{mol}^{-1}}{373 \mathrm{~K}}=108.3 \mathrm{~J} \cdot \mathrm{K}^{-1} \cdot \mathrm{mol}^{-1}\)
Class 11 Chemistry Notes For Entropy
Entropy change in an isothermal reversible expansion or compression of an ideal gas
Let, n mol of an ideal gas undergoes an isothermal reversible expansion from its initial state (P1 V1 to the final state (P2, V2). The equation showing the change in entropy of the gas in the process can be derived.
The result of this derivation gives the relation—
⇒ \(\Delta S=n R \ln \frac{V_2}{V_1}=2.303 n R \log \frac{V_2}{V_1}=2.303 n R \log \frac{P_1}{P_2}\)
As the gas expands, V2 > V2 (or, P1> P2 ), so according to the equation [1], the change in entropy (AS) of the gas due to its expansion is positive i.e., the entropy of the system increases.
If the isothermal reversible compression of the same amount of gas causes a change in the state of the gas form (P1 V1 to (P2, V2), then the change in entropy of the gas is given by
⇒ \(\Delta S=n R \ln \frac{V_2}{V_1}=2.303 n R \log \frac{V_2}{V_1}=2.303 n R \log \frac{P_1}{P_2}\)
As the gas is compressed, V2 < V1 (or P2> P1 ), According to equation [2], the change in entropy (AS) of the gas due to its compression is negative, i.e., the entropy of the system decreases.
Class 11 Chemistry Notes For Entropy
If the volume of a gas is increased, the gas molecules will get more space for their movement i.e., the gas molecules will move in greater volume. As a result, the randomness of the gas molecules as well as the entropy ofthe system (gas) will increase.
Thus, the entropy of a gas increases with the increase of its volume. On the other hand, the entropy of a gas decreases with the decrease of its volume.
MPBSE Class 11 Chemistry Notes For Change in entropy of the surroundings
When a system exchanges heat with its surroundings, the entropy of the system as well as its surroundings changes.
To calculate the change in entropy of the surroundings, the given points are to be considered:
Surroundings are so large compared to one system that they serve as a heat reservoir without undergoing any temperature change.
Surroundings absorb or release heat reversibly, and during these processes, the temperature and pressure of the surroundings remain almost the same In a process at TK, if the amount of heat released by the system to the surroundings is sys, then the amount of heat absorbed by tire surroundings = -guys (the sign of q is -ve).
Therefore, in this process, the change in entropy of the surroundings \(\Delta S_{s u r r}=-\frac{q_{s y s}}{T}\)
Class 11 Chemistry Notes For Entropy
Hence, the entropy of the surroundings increases if heat is released by the system to the surroundings.
In a process at T K, if the amount of heat absorbed by the system from the surroundings is q, then the amount of heat released by the surroundings will be -qsys (the sign of qsys is +ve ). So, in this process, the change in entropy of the surroundings, \(\Delta S_{\text {surr }}=-\frac{q_{s y s}}{T}.\)
Hence, the entropy of the surroundings decreases if heat is absorbed by the system from the surroundings
MPBSE Class 11 Chemistry Notes For Standard entropy change in a chemical reaction
Standard molar entropy of a substance:
Entropy of 1 mol of a pure substance at a given temperature (usually 25°C) &1 atm pressure is termed as the standard molar entropy of that substance.
It is denoted by S° and its unit is J. K-1.mol-1.
Standard entropy change in a chemical reaction (ΔS°):
In a chemical reaction, the change in standard entropy, ΔS° = total standard entropies of the products – total standard entropies of the reactants i.e.,
⇒ \(\Delta s^0=\sum n_i s_i^0-\sum n_j s_j^0\)
Where Soi and Soj are the standard entropy of the i -tit product and j -th reactant, respectively. n1 and n2 are the number of moles of the Mil product and i-th reactant, respectively In the balanced equation.
Class 11 Chemistry Notes For Entropy
In the case of the reaction.
⇒ \(a A+b B \rightarrow c C+a D\Delta S^0=\left(c S_C^0+d S_b^0\right)-\left(a S_A^0+b S_B^0\right)\)
MPBSE Class 11 Chemistry Notes For Change in entropy of the surroundings in a chemical reaction
The change In entropy of the surroundings in a given process,
⇒ \(\Delta S_{s t u r}=\frac{-q_{s y s}}{T},\) where qÿ is the heat absorbed by the system at 7’K.
In case of chemical reactions occurring at constant pressure \(q_{s y s}=q_P=\) change in enthalpy of the reaction system
⇒\(=\Delta H . \mathrm{So}_1, \Delta \mathrm{S}_{\text {surr }}=-\frac{\Delta H}{T}\)
For exothermic reactions,
⇒ \(\Delta H<0. \text { So, } \Delta S_{\text {surr }}=+v e \text {. }\) I-Ience, the entropy of the surroundings increases In an exothermic reaction.
For endothermic reaction
⇒ \(\Delta H>0 \text {. So, } \Delta S_{\text {surr }}=-v e \text {. }\) Hence, the entropy of the surroundings decreases in an endothermic reaction.
MPBSE Class 11 Chemistry Notes For Thermodynamic Process
Thermodynamic Process
Thermodynamic Process Definition:
A system is said to undergo thermodynamics. A pathway of process: The sequence of system a system process when it undergoes a processis called the pathways of process
Cyclic process Definition
A system is said to haw undergone a cyclic process I if it returns to Its initial state after a series of successive
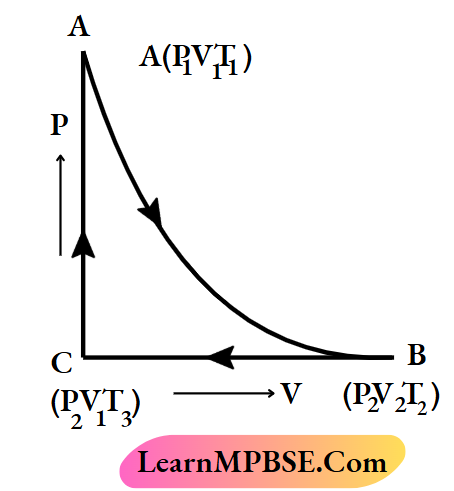
Cyclic process Explanation:
Let us consider a process, in which the initial state of a gaseous system is A (Pv Tx). The system returns to its initial state after undergoing consecutive returns to its initial state through three successive operations, it is a cyclic process.
Class 11 Chemistry Notes For Thermodynamic Process
Cyclic process Example:
The following change indicates a cyclic process because 1 mol of water (system) returns to its initial state again through successive changes
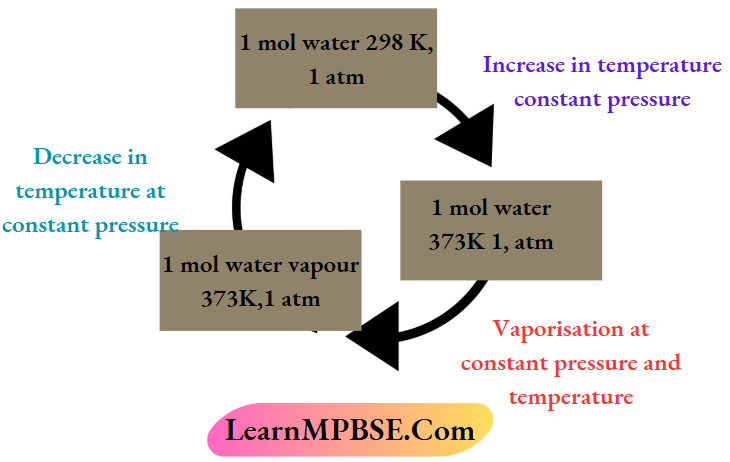
The changes in state functions are zero in a cyclic process. The value of a state function depends upon the present state of the system. Since the initial and the final states of the system are the same in a cyclic process, the rallies of the state functions in these two states are also the same. So die change in state function (ΔP, ΔV,ΔT,ΔU’, ΔH, etc.) becomes zero for a cyclic process
Isothermal process Definition
If the temperature of a thermodynamic system remains constant throughout a process, then the process Is said to be an Isothermal process,
At the time of conducting this process, the system Is kept lit contact with «a constant temperature heal hath (f.u, thermostat) with a high heat capacity. Such a heat hath Is capable of gaining or losing heat without changing Its temperature.
Condition(s) for the isothermal process:
During an Isothermal process, the temperature of the system remains constant. So we can write,dT ‘[system] = constant and d’V[system] or ΔT [system] = 0.
Class 11 Chemistry Notes For Thermodynamic Process
Condition(s) for the isothermal process Example:
The boiling of a liquid at its boiling point Is an isothermal process. This Is because the temperature of the liquid remains constant until the entire liquid converts to vapor. Thus, the boiling ofwater at 100°C and l atm is an isothermal process.
The temperature of the system remains fixed in the isothermal process. U does not mean that heat is not absorbed or liberated by the system during this process.
Isobaric process Definition
A process in which the pressure of the system remains fixed at each step of the process is called an isobaric process.
Condition(s)t for this and the isobaric process:
As the pressure of the system during this process remains constant P{system)=constant and dp (system)or ΔP (system) =0
Isobaric process Example:
The vaporization of any liquid in an open container occurs under atmospheric pressure. If the atmospheric pressure remains fixed, then the process of vaporization is said to be an isobaric process
Isochoric process Definition:
The isochoric process in Which the volume of the system remains constant throughout the process is called an isochoric to process.
Condition(s) for the isochoric process:
The volume of the system remains constant during this process. Hence, V[system] = constant and dV[system] or ΔV [system] =0
Isochoric process Example: The combustion of n substance In a bomb calorimeter.
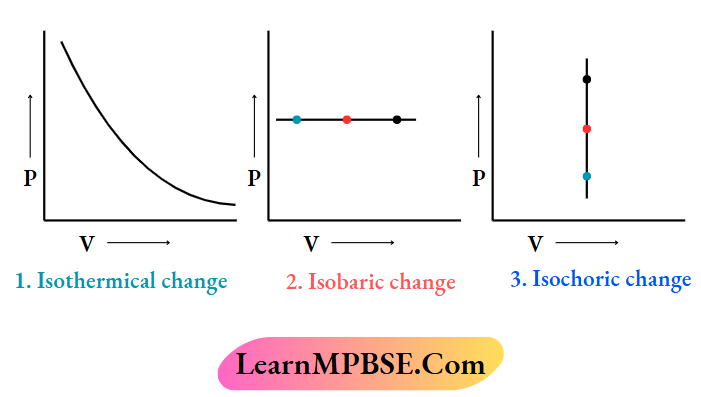
For a closed system consisting of an ideal gas. the plots of P vs V for
- Isothermic
- Isobaric and
- Isochoric changes
These are given below:
Class 11 Chemistry Notes For Thermodynamic Process
Adiabatic process Definition
A process in which no exchange of heat takes place between the system and Its surroundings at any stage of the process is called an adiabatic process.
This process requires the system to be covered with a perfect thermal insulator. But in reality, no such material is available and hence the process never becomes one hundred percent adiabatic.
Condition(s) for adiabatic process:
No heat is exchanged between a system and its surroundings In an adiabatic process. So, for such type of process q – 0; where q = heat absorbed or released by the system.
Adiabatic process Example:
The sudden expansion or compression of a gas is considered to be an adiabatic process because when a gas (system) undergoes such a process, it does not get a chance to exchange heat with its surroundings. As a result, the sudden compression of a gas results in an increase in the temperature of the gas, while its sudden expansion leads to a decrease in temperature. For example, when the valve ofa bicycle or car tire is removed, the air coming out ofthe tire undergoes very fast expansion and gets cold.
- The temperature of a system does not remain constant in an adiabatic process (except in the case of an adiabatic expansion of an Ideal gas against zero pressure).
- For a process involving more than one step, the algebraic sum of heat absorbed or released in different steps may be zero q1 + q2 + q3 + ……………….. = 0, but it does not mean that the process is an adiabatic one.
Reversible process Definition
A process It salt! to be reversible If It Is carried out Infinitesimally slowly so that In each step the thermodynamic equilibrium of the system Is maintained, and any Infinitesimal change In conditions can reverse the process to restore both the system and Its surroundings lo their Initial states.
Class 11 Chemistry Notes For Thermodynamic Process
Reversible process Explanation:
Let us consider that the gas (system) Is enclosed lit a cylinder lilted with a weightless and frictionless piston and the cylinder is kept At a constant temperature hath (surroundings). So, any change occurring In the ire system would be Isothermal. Since the temperature of the system and surroundings tiro the same, the system will remain In thermal equilibrium.
Let the applied external pressure acting on the piston Is the same as that of the gas. So, the system will remain In mechanical equilibrium. Hence, In this condition, the system, the gas Is In a state of thermodynamic equilibrium and pthe roperties of the system remain unchanged
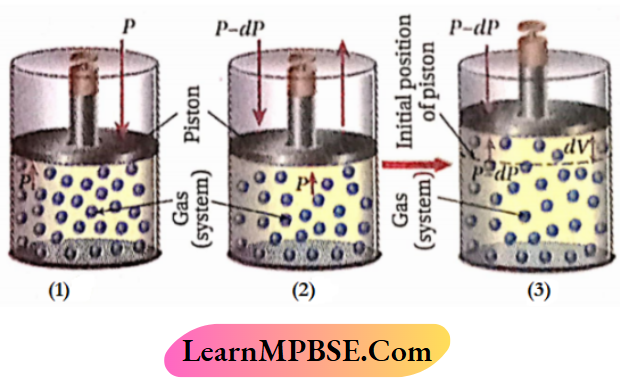
Now, if the external pressure is decreased by an infinitesimal amount dP, the volume of the gas will increase very slowly by an infinitesimal amount until the pressure of the gas becomes equal to that of the external pressure.
- Let the infinitesimal increase of volume = dV. If the external pressure is further decreased by an Infinitesimal amount of dP, the volume of the gas will also increase by an infinitesimal amount of dV.
- In this way, if the gas is allowed to expand very slowly in an infinite number of steps by decreasing an infinitesimal amount (dP) of the external pressure at each step, then the expansion ofthe gas will reversibly take place.
- In a step, if the external pressure is increased by an infinitesimal amount (dP), then the volume of the gas will decrease by an infinitesimal amount dV.
- Hence, by decreasing or increasing the external pressure by an infinitesimal amount, the direction of the process can be reversed.
Class 11 Chemistry Notes For Thermodynamic Process
Characteristics of a reversible process:
- The driving force of a reversible process is infinite¬ simply greater than the opposing force and by increasing or decreasing the driving force by an infinite¬ amount, the direction of the process can be changed.
- In this process, the system remains in thermodynamic equilibrium at every intermediate step. This process is extremely slow. From a theoretical point of view, any reversible process requires infinite time for its completion.
- After the completion a reversible process, if the system is made to return to its initial state by traversing the forward sequence of steps in the reverse order, then both the system and its surroundings are restored to their initial states.
- The work done by a system in a reversible process is always the maximum
- The reversible process is extremely slow and infinite time is required to complete the process. A true reversible process is a hypothetical concept. In practice, no process can be carried out reversibly.
- All processes occurring in nature (i.e., real processes) are irreversible. Nevertheless, the concept of reversibility has immense importance in thermodynamics
Examples of some reversible processes:
1. The vaporization ofa liquid at a particular temperature in a closed container can be considered as a reversible process. Let us consider a certain amount of water is in equilibrium with its vapour at T K temperature in a closed container. Here water and water vapour together constitute a system.
- If the temperature of the system is increased by an infinitesimal amount of dT, then a very small amount ofwater will vaporize and a new equilibrium will be established.
- Consequently, the vapor pressure of water will be increased by an infinitesimal amount of DP. If the temperature of the system is decreased by an infinitesimal amount of dT, the same amount of water vapour will condense to establish equilibrium.
- As a result, the vapour pressure of water is also decreased by an infinitesimal amount of dP. So, by increasing or decreasing the driving force (i.e., by increasing or decreasing temperature) the direction of the process can reversed.
- Thus, the vaporisation of a liquid at a particular temperature in a closed vessel approximates to a reversible process.
2. Reaction occuring in a galvanic cell is reversible in nature.
- If an external potential applied between the two electrodes is slightly less in magnitude but opposite in sign than the electromotive force (EMF) of the cell, then the direction of flow of the current and the cell reaction remains unaltered.
- But, if the externally applied potential slightly exceeds the EMF ofthe cell in magnitude, then the direction of the cell reaction and the direction of current are found to be reversed.
- Therefore, by slightly increasing or decreasing the external potential (with respect to EMF of the cell), the direction of the cell reaction can be changed.
- Thus, the reaction occuring in a galvanic cell approximates to a reversible process.
Class 11 Chemistry Notes For Thermodynamic Process
Irreversible process Definition
Aprocess which occurs at a finite rate with the finite ite changes in properties of the system, and at any stage during the process, the system cannot get a chance to remain in thermodynamica equilibrium is called an irreversible process
All natural processes are irreversible in nature.
Irreversible process Explanation:
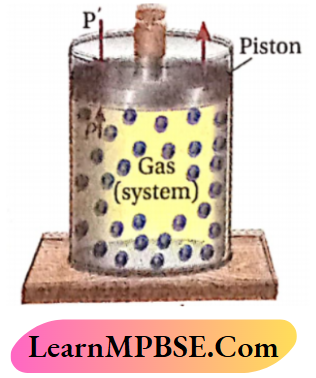
Let us consider that a certain amount of a gas is enclosed in a cylinder fitted with a weightless and friction¬less piston and the cylinder is kept in a constant temperature heat-bath (thermostat).
- Therefore ,the temperature of the system gas becomes equal to that of the (system) tsurroundings (thermostat).
- Let the external pressure applied on the piston (P) and the pressure of the gas are the same
- Now, if the external pressure is suddenly reduced to P’ , then the gas will expand at a finite rate and this will be irreversible because during expansion the system does not maintain in thermodynamic equilibriuig
Characteristics of an irreversible process:
In an irreversible process there is an appreciable difference between the driving force and the opposing force (actually, the driving force is greater than the opposing force).
- As a result, such a process takes place at a finite rate, although sometime a very’ slow process may also be irreversible in nature.
- In an irreversible process, the system does not remain in thermodynamic equilibrium at any stage during the Examples: O The flow of heat from an object of higher process.
- If an irreversible process is reversed and the system is made to go back to its initial state, then the work done in the backward direction will not be the same as that in the forward direction
- After the completion of an irreversible process, although the system can be brought back to Its initial state, its surroundings cannot be.
- Irreversible processes complete in a finite time.
Irreversible process Examples: The flow of heat from an object of higher temperature to an object of lower temperature.
- The downward flow of water from a mountain.
- The expansion of a gas against zero pressure.
- The formation of curd from milk
- Differences between the reversible and irreversible processes
Class 11 Chemistry Notes For Thermodynamic Process
Differences between the reversible and irreversible processes:
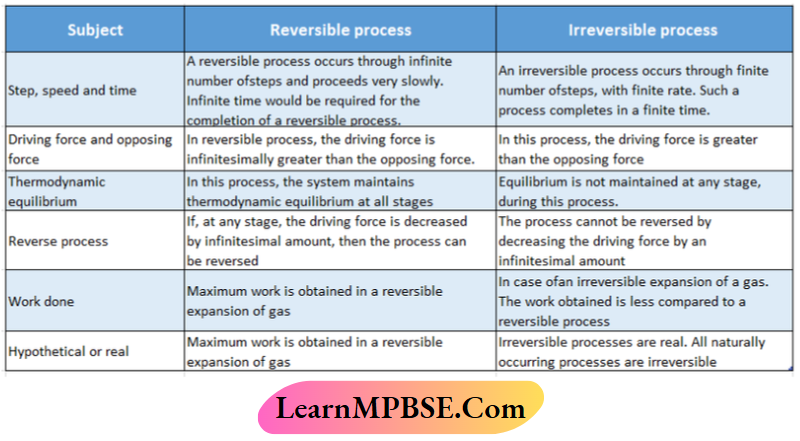
Differences between the reversible and irreversible processes:
MPBSE Class 11 Chemistry Notes For Thermodynamic Properties
Thermodynamic Properties And Thermodynamic State Of A System
Thermodynamic properties
The measurable physical quantities by which thermo¬ dynamic state ofa system can be defined completely are called thermodynamic properties or variables ofthe system.
Thermodynamic properties Examples:
The pressure (P), temperature (T), volume (V), composition etc., of a system are the thermodynamic properties or variables of the system because the state of the system can be defined by these variables or properties. The properties or variables required to define the state ofa system are determined by experiment.
- Although a thermodynamic system may have many properties (like— pressure, volume, temperature, composition, density, viscosity, surface tension, etc.), to define a system we need not mention all of them since they are not independent
- If we consider a certain number of properties or variables having certain values to define the state of a system, then the other variables will automatically be fixed. In general, to define the state of a thermodynamic system, four properties or variables are needed. These are pressure, volume, temperature and composition of the system.
- If these variables of a thermodynamic system are fixed then the other variables will also be fixed for that system. For a closed system of fixed composition, the state of the system depends upon pressure (P), temperature (T) and volume (V) of the system.
- If these three variables of the system (P, V, T) are fixed, then other variables (like density, viscosity, internal energy etc.) ofthe system automatically becomes fixed
Class 11 Chemistry Notes For Thermodynamic Properties
Thermodynamic state of a system
A system is said to be in a given thermodynamic state ifthe properties (e.g., pressure, volume, temperature etc.) deter¬ mining its state have definite values.
If the thermodynamic properties or variables of a thermodynamic system remains unchanged with time, then the system is said to be in thermodynamic equilibrium.
A system is said to be in thermodynamic equilibrium if it attains thermal equilibrium, mechanical equilibrium, and chemical equilibrium simultaneously.
- Thermal equilibrium: A system is said to be in thermal equilibrium if the temperature throughout the system is the same and is equal to that of its surroundings.
- Mechanical equilibrium: If no imbalanced force exists within a system and also between the system and its surroundings, the system is said to be in mechanical equilibrium.
- Chemical equilibrium: If the chemical composition throughout a system remains the same with time, the system is said to be in chemical equilibrium

State function of a thermodynamic system definition
A state function of a thermodynamic system is a property whose value depends only on the present state of the system but not on how the system arrived at the present state.
Thermodynamic System Examples:
Pressure (P), volume (V), temperature (T) , internal energy (E or U), enthalpy (H), entropy (S), Gibbs free energy (G) etc., of a thermodynamic system are the state functions because the values of these functions depend only on the present state ofa system, not on how the system arrived at that state.
Class 11 Chemistry Notes For Thermodynamic Properties
Thermodynamic System Change of a state function in a process:
The state of a thermodynamic system at the beginning of a process is called its initial state and the state attained by the system after completion of the process is called its final state. Let X (like P, V,T etc., of a system) be a state function of a thermodynamic system. The values of X at the beginning and at the end of a process are X1 and X2 respectively. So, the change in the value of X in the process, ΔX = X2-X1
Infinitesimal change in X is represented by dX and finite change in X is represented by AX. For example, the infinitesimal change in pressure (P) of a system is dP and the finite change is ΔP.
If X is a state function of a thermodynamic system, then dX must be a perfect differential as the integration of dX between two states results in a definite value of X
The state function of a system is a path-independent quantity:
A state function of a system depends only on the state of the system. Consequently, the change in any state function ofa system undergoing a process depends only upon the initial and final states of the system in the process, not on the path of the process. Thus the state function of a system is a path-independent quantity
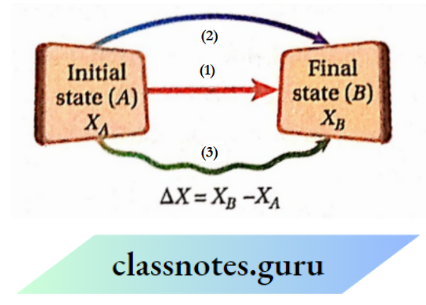
Thermodynamic System Explanation:
Suppose, a system undergoes a process in which its state changes from A (initial state) to B (final state), and because of this, the value of its state function X changes from XA (value of X at state A ) to XB (value of X at state B). The process can be carried out by following three different paths as shown
But the change in X, i.e., ΔX= (XB-XA) will be the same for all three paths. This is because all the paths have identical initial and final states and consequently X has identical initial and final values for these paths.
Class 11 Chemistry Notes For Thermodynamic Properties
Thermodynamic System Example:
The change in temperature of a system depends only upon the initial and final states of the process. It does not depend on the path followed by the system to reach the final state. So the temperature of a system is a state function. Similarly, the change of other state functions like pressure (P), volume (V), internal energy (U), enthalpy (H), entropy (S), etc., (i.e. ΔP, ΔV, ΔU, ΔH, ΔS etc.) does not depend upon the path ofthe process.
Path-dependent quantity
Two terms commonly used in thermodynamics are heat (q) and work ( w). These are not the properties ofa system. They are not state functions. Heat change or work done involved in a process depends on the path of the process by which the final state of the system is achieved. Thus, heat and ; work are the path-dependent quantities.
In general, capital letters are used to denote the state functions (for example, P, V, T, U, etc.) and small letters are used to denote path functions (for example q , w, etc.). q and w are not state functions. Hence, δq or δw (S = delta) are used instead of dq or dw. Unlike dP or dV, which denotes an infinitesimal change in P or V, δq or δw does not indicate such kind of change in q or w. This is because q and w like P or F are not the properties of a system. δq and δw are generally used to denote the infinitesimal transfer of heat and work, respectively, in a process.
MPBSE Solutions For Class 11 Chemistry Chapter 6 Chemical Thermodynamics
MPBSE Solutions For Class 11 Chemistry Chapter 6 Chemical Thermodynamics Question And Answers
Question 1. Give two examples of path-dependent quantities. Are they properties ofa system?
Answer:
Two path-dependent quantities are heat (q) and work ( w). These are not the properties ofa system.
Question 2. Under what conditions will a system be in thermodynamic equilibrium?
Answer:
A system will be in thermodynamic equilibrium if it simultaneously maintains mechanical equilibrium, thermal equilibrium and chemical equilibrium.
Solutions For Class 11 Chemistry Chapter 6 Chemical Thermodynamics
Question 3. Why is a process occurring in an open container considered to be an isobaric? What is the origin of the internal energy of a system? Why cannot the absolute value of internal energy be determined?
Answer:
A process in an open container takes place under constant atmospheric pressure. Thus, it is an isobaric process.
Read and Learn More Class 11 Chemistry
Question 4. Is the internal energy of a system at 25°C greater or less than its internal energy at 50°C?
Answer:
The internal energy of a system increases with the temperature rise. So, the internal energy ofa system will be greater at 50 °C than that at 25 °C.
Question 5. Under which condition will the pressure-volume work be, \(w=-\int_{V_1}^{V_2} P d V?\) pressure of the gas.
Answer:
⇒ \(w=-\int_{V_1}^{V_2} P_{e x} d V\)
So, in case ofa reversible process, \(w=-\int_{V_1}^{V_2} P d V\)

Question 6. According to the first law of thermodynamics, AU = q + w. Write down the form of this equation for the following processes: Cyclic process Adiabatic process Isothermal expansion of an ideal gas Process occurring in an isolated system.
Answer:
An isolated system does not exchange energy or matter with its surroundings. So, for a process occurring in an isolated system, q = 0 and w = 0. Therefore, ΔU = q + w or, ΔU= 0 + 0 or, ΔU = 0
Solutions For Class 11 Chemistry Chapter 6 Chemical Thermodynamics
Question 7. The definition of enthalpy shows that for n mol of an ideal gas H = U + nRT.
Answer:
If the enthalpy, internal energy, pressure and volume of ‘n’ mol of an ideal gas at a temperature of TK are, U, P and V respectively, then H = U+PV. For the ‘n’ mol of an ideal gas, PV = nRT. So, H = U+nRT.
Question 8. Prove that for an Ideal gas undergoing an isothermal change, AH = 0.
Answer:
The change in enthalpy of an ideal gas undergoing a process, ΔH = ΔU+nRAT. In an isothermal process, ΔT = O. So, ΔH = A{Again, in an isothermal process of an ideal gas, A U = 0 and hence AH = 0.
Question 9. Under what conditions are
- ΔU = qv
- ΔH = qp
Answer:
ΔU = qv; Conditions: Closed system, constant volume, only P-V work is considered
ΔH = qp; Conditions: Closed system, constant pressure, only P-V work is considered.
Question 10. Give an example ofa combustion reaction whose standard enthalpy change is equal to the standard enthalpy of formation ofthe compound formed in the reaction.
Answer:
At 25 C the standard heat of combustion of solid naphthalene [C10H8(s)] is 5147 kj. mol-1. this means that at 25 c and 1 atm pressure when 1 mol of solid naphthalene is completely burnt in the presence of oxygen the enthalpy change that occurs is 51747kj.
Solutions For Class 11 Chemistry Chapter 6 Chemical Thermodynamics
Question 11. The standard enthalpy of combustion of CxHy) at 25°C is Q kj. mol-1 . Write down the thermochemical equation for the combustion reaction of this compound.
Answer:
The combustion reaction for C(s, graphite) is:
This reaction also C(s, graphite) + O2(g)→CO2(g). represents the formation reaction of CO2(g). Therefore, at 25°C, the standard heat of combustion of C(s, graphite) = the standard heat formation of CO2(g)
Question 12. Consider the given enthalpy diagram, and > calculate the unknown AH by applying Hess’s law.
Answer:
Following the given diagram, we have
- A+B→ C + 2D ; ΔH = ?
- A + B→ E + 2D; ΔH = +27kJ
- E + 2D →C+ 2D;ΔH = -13kJ
Adding equation 2 and equation 3, we get A + B→C+2D; ΔH = (27- 13)kJ
= 14 kJ
Solutions For Class 11 Chemistry Chapter 6 Chemical Thermodynamics
Question 13. For the reaction, A + B →D, AH is -30 kj. Suppose, D is prepared from A and B and then it is again converted into A and B by following the stages D → E → A + B. Calculate the total enthalpy change in these two stages.
Answer:
A + B → D; ΔH = -30 kj ……………………….(1)
The process D→E →A + B comprises the following two steps:
D→E ⋅⋅⋅⋅⋅(2) and E→A + B
Overall reaction: D→A + B The reaction (4) is the opposite ofthe reaction (1).
So, the enthalpy change in reaction (4) is +30 kj.
Hence, the total enthalpy change in steps (2) and (3) is +30 kJ
Question 14. Water remains in equilibrium with its vapour at 100°C and atm. Will the transformation of water into its vapour be spontaneous at this pressure and temperature?
Answer:
No. Since water and its vapour are in equilibrium, neither the forward process (water→vapour) nor the reverse process (vapour →water) is favourable
Question 15. If the process A→B occurs reversibly, then the change in entropy of the system is ΔS1. When the same process occurs irreversibly, the change in entropy of the system is ΔS2. Will the value of ΔS1 be greater than, less than or equal to the value of ΔS2?
Answer:
Since entropy is a state function, the change in entropy ofa system in a process does not depend upon whether the process is carried out reversibly or irreversibly.
Solutions For Class 11 Chemistry Chapter 6 Chemical Thermodynamics
Question 17. Write the relation between ΔSsys & ASsurrwhen a process reaches equilibrium. What will be the value of ΔSuniv?
Answer:
For a process at equilibrium
⇒ \(\Delta S_{\text {system }}+\Delta S_{\text {surr }}=0\text { But } \Delta S_{s y g}+\Delta S_{\text {surr }}=\Delta S_{\text {univ }} \text {. So, } \Delta S_{\text {univ }}=0\)
Question 18. Give two examples of spontaneous processes in which the disorderliness of the system decreases.
Answer:
Transformation of water into ice at 1 atm pressure and below (T’C temperature. Condensation of water vapor at1 atm pressure and below 100 C temperature.
Question 19. What do you mean by a perpetual motion machine of the second kind?
Answer:
A machine working in a cyclic process absorbs heat from a single thermal reservoir and completely converts the heat into the equivalent amount of work, is called a perpetual motion machine of a second kind. This type of machine contradicts the second law of thermodynamics, & it is impossible to construct.
Question 20. A certain amount of gas is enclosed in a container with permeable and diathermal walls. Which type of system does the gas belong to?
Answer:
The walls of the container are diathermal. So the system can exchange heat with its surroundings. Again, the walls ofthe container are permeable. So, the system can also exchange matter with its surroundings. Hence, the gas belongs to an open system.
Solutions For Class 11 Chemistry Chapter 6 Chemical Thermodynamics
Question 21. Does the volume of a closed system remain fixed?
Answer:
If the walls of a closed system are non-rigid or movable, then the volume of the system does not remain fixed. For example, a gas enclosed in a cylinder fitted with a movable piston is considered a closed system. Here, the volume of the gas (system) can be increased or decreased by altering the pressure of the gas (system)
Question 22. Give an example of a thermodynamic quantity which is not a state function. Is it a property of a system?
Answer:
Heat is not a state function because heat absorbed or by a system in a process depends upon the path of the realised It is not a property of the system.
Question 23. Give an example of a process which Is simultaneously isothermal and adiabatic.
Answer:
Adiabatic free expansion of an ideal gas (or isothermal free expansion of an ideal gas). The reason is that no exchange of heat occurs between the system and surroundings in this process and the temperature of the tire system remains constant throughout the process.
Question 24. At 25°C, the standard reaction enthalpy for the reaction AB3(g)→1/2A2(g)+3/2(g) is. find the standard reaction enthalpy for the reaction.
Answer:
Writing this equation. in reverse manner and multiplying both sides by 2, we get,
A2(g) + 3B2(g)→2AB3(g); -2AH°. So, at 25°C, the standard
Question 25. Mention the standard state of sulphur and iodine at 25
Answer:
At 25°C, the standard state of sulphur is solid rhombic sulphur [S(rhombic, s)] and that of iodine is solid iodine [12(s)].
Question 26. What do you mean by ‘the enthalpy of solidification of water at 0°C and 1 atm pressure = -6.02 kj-mol-1 .’?
Answer:
This means that 6.02 kj of heat is released when one mole of water completely freezes to ice at 0°C and 1 atm pressure.
Question 27. Why are spontaneous natural processes irreversible?
Answer: ‘
The spontaneous or natural processes are irreversible because the thermodynamic equilibrium of the system is not maintained in such types of processes.
Solutions For Class 11 Chemistry Chapter 6 Chemical Thermodynamics
Question 28. Which of the following will have a greater entropy?
- 1 mol of H2 gas (T = 300 K, V = 5ml, )
- 1 mol of H2 gas (T = 300 K, V = 10mL).
Answer:
As the entropy of a gas increases with the increase in its volume,
- The entropy of mol of H2 (T = 300 K, V = 10 mL)
- Will be greater than that of l mol of H2 (T = 300 K, V = 5 mL).
Question 29. For a process, ΔSsys = -15 J.K-1 .. For what value of ASsurr will the process be non-spontaneous?
Answer:
The condition of non-spontaneity of a process is \(\Delta S_{s y s}+\Delta S_{s u r r}<0.\) \(\Delta S_{s y s}=-15 \mathrm{~J} \cdot \mathrm{K}^{-1}\), then will be non-spontaneous
Question 30. A gas is allowed to expand against zero external pressure. Explain with reason whether the process is reversible or irreversible.
Answer:
The expansion ofa gas against zero external pressure is an irreversible process. As the opposing pressure is zero, the gas expands rapidly, and it cannot maintain thermodynamic equilibrium during its expansion.
Question 31. In a process, 701 J of heat is absorbed by a system and 394J of work is done by the system. What is the change in internal energy for the process?
Answer:
Given: q = +701J and w = -394 J {-ve sign as the work is done by the system)
Now, ΔU = q + w or, A U = (701- 394)J = +307 J.
So, the change in internal energy of the system = +307 J.
Question 32. The latent heat of the vaporization of water at a normal boiling point is 40.75 kJ. mol-1 . . Calculate the change in entropy of vaporization.
Answer:
Given:
⇒ \(\Delta H_{\text {vap }}\) = 4075 kJ. mol-1,
Tb =100C = 375k
⇒ \(\Delta S=\frac{\Delta H_{v a p}}{T_b}=\frac{40.75 \times 10^3 \mathrm{~J} \cdot \mathrm{mol}^{-1}}{373}=109.25 \mathrm{~J} \cdot \mathrm{mol}^{-1}\)
Question 33. Due mole of ideal gas is expanded isothermally. In this process, which of the quantity (or quantities) among w. q, ΔH , ΔH is(are) zero or >0 or <0?
Answer:
During isothermal expansion, heat is absorbed and work is done by the gas. So q > 0 and w < 0. Again internal energy and enthalpy remain the same during isothermal expansion of an ideal gas. Thus, ΔU = 0 and ΔH = 0 . For the isothermal expansion of mol of ideal gas q>0, w< 0, ΔU = 0, ΔH = 0.
Solutions For Class 11 Chemistry Chapter 6 Chemical Thermodynamics
Question 34. Give examples of two processes by which the internal energy of a gas can be increased.
Answer:
The internal energy of a gas can be increased by increasing the die temperature ofthe gas. If a gas is compressed adiabatically (considering only P-V work) its internal energy increases.
Question 35. Give examples of three processes in which the change in internal energy of the system is zero.
Answer:
The change in internal energy of any cyclic process is zero. The change in internal energy of an ideal gas during isothermal expansion or compression is zero. In adiabatic free expansion of an ideal gas, the change in internal energy is equal to zero.
Question 36. What do you mean by the standard enthalpy of atomisation of chlorine at 25°C = + 121 kj.mol-1 .?
Answer:
This means that at 25°C and 1 atm pressure, 121 kj of heat is required to produce 1 mol of gaseous Cl-atom from Cl2(g). Thus, the change in enthalpy for the process,
⇒ \(\frac{1}{2} \mathrm{Cl}_2(\mathrm{~g}) \rightarrow \mathrm{Cl}(\mathrm{g}); \Delta H_{\text {atom }}^0=+121 \mathrm{~kJ}\)
Solutions For Class 11 Chemistry Chapter 6 Chemical Thermodynamics
Question 37. When does the entropy of the system attain maximum value for a spontaneous or irreversible process occurring in an isolated system? Under this condition, what will be the change in entropy of the system?
Answer:
When a spontaneous or irreversible process occurs in an isolated system, the entropy of the system increases with the progress of the process towards equilibrium. The value of entropy becomes maximum when the process attains equilibrium, and there occurs no further change in the entropy ofthe system. Thus, the value of entropy is maximum at the equilibrium state ofthe process and under this condition, the change in entropy ofthe system is zero.
Question 38. Is the entropy change of a system influenced by the change in temperature? Explain.
Answer:
The entropy of a system is highly dependent on temperature. With the increase or decrease in temperature, the randomness of the constituent particles (atoms, molecules or ions) of a system increases or decreases. Now, the entropy of a system is a measure of the randomness of its constituent particles. Thus, the entropy change ofa system is influenced by the change in temperature.
MPBSE Solutions For Class 11 Chemistry Chapter 7 Equilibrium
MPBSE Class 11 Chemistry Chapter 7 Equilibrium Question And Answers
Question 1. A liquid is in equilibrium with its vapor at its boiling point. On average which property of the molecules is equal in two phases?
Answer:
At the boiling point of a liquid in equilibrium with its vapor, the average kinetic energy of the molecules in the two phases is equal.
Question 2. According to Le Chatelier’s principle, what is the effect of adding heat to a solid and liquid in equilibrium?
Answer:
In the equilibrium system solid-liquid, the forward process is endothermic. Therefore, if temperature is increased at equilibrium, then, according to Le Chatelier’s principle, equilibrium will shift to the right, thereby increasing the amount of liquid.
Question 3. Mention two ways by which the equilibrium of the L-given reaction can be shifted to the right.
Answer:
According to Le Chatelier’s principle, the equilibrium of the above reaction can be shifted to the right by the addition of excess reactants [i.e., CH3COOH(l) or C2H5OH(l) ] or by the removal of the products [i.e., CH3COOC2H5(l) or H2O(l)] from the reaction system at a given temperature, keeping the volume of the reaction system constant.
Read and Learn More Class 11 Chemistry
Question 4. When steam is passed over a red-hot iron, H2 gas is produced. In this reaction, the yield of H2(g) is found to increase when the partial pressure of steam is increased. Explain.
Answer:
Reaction:
⇒ \( \mathrm{Fe}(s)+4 \mathrm{H}_{-} \mathrm{O}(\rho) \rightleftharpoons \mathrm{Fe}_{-} \mathrm{O} \cdot(\mathrm{s})+4 \mathrm{H}_{-}(g)\)
A steam is one of the reactants in the above reaction, increasing its partial pressure at equilibrium will shift the equilibrium position to the right. As a result, the yield of the product i.e., H2(g) will increase.
Class 11 Chemistry Chapter 7 Equilibrium
Question 5. Why does not the equilibrium constant expression for a reaction involving pure solids or liquids contain the concentration terms of the solids or liquids?
Answer:
For a pure solid or liquid, molar concentration is directly proportional to density. Given that density remains constant at a specific temperature, the molar concentration of a pure solid or liquid at that temperature is a constant value, typically regarded as unity (1). Consequently, the equilibrium constant expression for a process involving pure solids or liquids excludes concentration terms for these phases.

Question 6. At constant temperature, the following reaction is at equilibrium in a closed container: \(\mathrm{C}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightleftharpoons \mathrm{CO}(\mathrm{g})+\mathrm{H}_2(\mathrm{~g})\) At constant temperature, if the amount of the solid carbon is reduced to half at equilibrium, then what will be the change in the concentration of CO(g)?
Answer:
At a particular temperature, the concentration of any pure solid is independent of its amount. Thus, keeping the temperature constant, if the amount of solid carbon is reduced to half at equilibrium, then its concentration will remain unchanged. So, the concentration of CO(g) will also remain unaffected.
Class 11 Chemistry Chapter 7 Equilibrium
Question 7. The values of the equilibrium constant (if) of a reaction at 25°C & 50°C are 2 ×10-4 & 2 ×10-2, respectively. Is the reaction exothermic or endothermic?
Answer:
With the increase in temperature, the value equilibrium constant (K) increases for an endothermic reaction, while it decreases for an exothermic reaction. For the reaction, K(50°C) > K(25°C), indicating it is an exothermic reaction.
Question 8. For a gaseous reaction, Kp > Kc. What will be the effect on equilibrium if pressure is increased at a constant temperature? Will it affect the yields of the products?
Answer:
According to the relation Kp = Kc(RT)-Δn, if Kp> Kc, then Δn > 0. The positive value of An implies that the reaction occurs with an increase in volume in the forward direction. For such a reaction, if pressure is increased at equilibrium, then according to Le Chatelier’s principle the equilibrium will shift to the left and thus the yield ofthe product will decrease.
Question 9. In the case of the thermal decomposition of H2(g) to H(g), which conditions of pressure and temperature will be favorable for an increase in the yield of H(g)?
Answer:
Since the formation of H(g) from H2(g) [H2(g) 2H(g)] occurs through decomposition, it is an endothermic reaction. Because 2 moles of H(g) are formed from 1 mole of H2(g), the reaction is associated with a volume increase. So, according to Le Chateliehs principle, the yield of H (g) will increase if the reaction is carried out at a high temperature and low pressure.
Class 11 Chemistry Chapter 7 Equilibrium
Question 10. In the case of the reaction A2(g) + 4B2(g), 2AB4(g), the change in enthalpy (ΔH) is negative. Mention the conditions of pressure and temperature at which the yield of the product, AB4(g) will decrease.
Answer:
Since ΔH < 0, it is an endothermic reaction. The volume of the reaction system decreases in the forward direction [1 molecule of A2(g) combines with 4 molecules of B2(g) to form 2 molecules of AB4(g)]. Thus; according to Le Chatelier’s principle, under the conditions of high temperature and low pressure, the yield of AB4(g) will decrease.
Question 11. How will the equilibrium of the reaction, H2(g) + I2(g) ⇌ 2HI(g) be affected if the volume of the reaction system at equilibrium is doubled, keeping the temperature constant?
Answer:
Doubling the volume of the reaction system at equilibrium will reduce the total pressure of the system by half. But for the reaction Δn = 0. So, according to Le Chatelier’s principle, the equilibrium ofthe reaction will not be affected by a change in pressure.
Question 12. In the reaction, I2+I–→I3–, which one acts as a Lewis base?
Answer:
In the reaction between and I2, the I– ion donates an electron pair to the I2 molecule, resulting in the formation of the I3– ion [I2+I–→I3– ]Therefore, the I– ion acts as a Lewis base in this reaction.
Question 13. The pKa values of the three weak acids HA, HB, and HC are 4.74, 3.75, and 4.20, respectively. Arrange them in order their of increasing acid strengths.
Answer:
As pKa = -log10 Ksp, the smaller the value of Ka the larger the value of pKa. So, an acid with a larger pKa will have a smaller Ka. As, pKa(HB) < pKa(HC) < pKa(HA) , pKa(HB) > Kfa(HC) > Ka(HA). At a certain temperature, a higher value of Ka for an acid indicates a higher strength of the acid. Therefore, the increasing order of acid strengths of the given acids will be — HA < HC < HB.
Question 14. X and Y are two aqueous solutions of added HA with concentrations of 0.1 M & 0.01M, respectively. In which solution will the degree of ionization of U A be higher
Answer:
According to Ostwald’s dilution law, the degree of ionization of a weak electrolyte increases with the increase in dilution of its aqueous solution. Since, the concentration of solution Y is less than that of X, the degree of ionization of HA will be higher in solution Y.
Class 11 Chemistry Chapter 7 Equilibrium
Question 15. Which one of the following two acids will have a higher concentration of H3O+ ions in their 0.1(M) aqueous solutions HCl and CH3COOH?
Answer:
HCl is a strong acid, while CH6COOH is a weak acid. Thus, HCl ionizes almost completely in aqueous solution, whereas CH3COOH undergoes partial ionization. As a result, the concentration of H30+ ions in 0.1(M) HCl solution is higher than that in 0.1(M) CH3COOH solution.
Question 16. Show that [OH-]>\(\sqrt{K_w}\) in an alkaline solution.
Answer:
We know, [H3O+] × [OH– ] = Kw. In pure water, [H3O+] = [OH–].
This gives \(\left[\mathrm{H}_3 \mathrm{O}^{+}\right]=\left[\mathrm{OH}^{-}\right]=\sqrt{K_w}\)
In an alkaline solution, the concentration of OH– ions is higher than that in pure water.
Therefore, in an alkaline solution, \(\left[\mathrm{OH}^{-}\right]>\sqrt{K_w} \text {. }\).
Question 17. Will the concentration of HgO+ ions in pure water at 0°C be more than or less than that at 4°C?
Answer:
Ionization of water is an endothermic t process: [2H2O(1) H3O+(aq) + OH–(aq)]. Hence, with, a temperature rise, the ionic product of water (Kw) increases. Therefore, KM,(4°C) In pure water, [H3O+] = Jÿw- Since,(4°C) > Kw(0°C) , the concentration of H2O+ ions in pure water at 4 °C will be higher than that at 0°C.
Question 18. At a certain temperature, what is the value for the die sum of pH and pOH for an aqueous solution? What will be its value at 25°C?
Answer:
In case of any aqueous solution at a certain temperature pH+POH=pkw. At 25C, Pkw= 14. Hence at 25C PH+POH=14.
Class 11 Chemistry Chapter 7 Equilibrium
Question 19. An acid bottle labeled pH – 5 Is this acid a weak acid?
Answer:
The acid may be a weak add or a very dilute strong acid. The pH of an added solution depends upon the die concentration of H3O+ Ions in the solution. So, from the value of pH, it Is not possible to predict whether the acid IN is weak or strong.
Question 20. A, B, and C are three buffer solutions, each of which is composed of a weak acid and its salt. For increasing the pH by 0.02 units, it is found that 1.0, 1.4, and 1.2 millimol of NaOH are required for A, B, and C, respectively. Arrange the solutions in the increasing order of their buffer capacities.
Answer:
The higher the buffer capacity of a buffer solution, the greater the amount of a strong acid or a strong base to be required for increasing the pH of the buffer. It is given that increasing the pH of the buffer by the same amount requires a minimum amount of NaOH for buffer A and a maximum amount of NaOH for buffer B. Therefore, the increasing order of buffer capacity of the given buffers is A < C < B.
Question 21. Of the two bottles, one contains an HCl solution and the other a buffer solution. Each of the bottles b labelled as pH = 5. How can you identify the solutions?
Answer:
Upon measuring the pH of the solutions following the addition of equal drops of NaOH, a significant increase in pH will be observed for one solution, whilst the pH of the second solution remains relatively unchanged. A buffer solution maintains a relatively constant pH, whereas a solution of HCl results in an increase in pH.
Question 22. At a certain temperature, the Ksp of AgCl in water is 1.8 × 10-10. What will be its Ksp is a 0.1M solution of AgNO3 at some temperature.
Answer:
At a certain temperature, the solubility of AgCl decreases in the presence of a common ion (Ag+), but the solubility product of AgCl remains the same. Therefore, Ksp for AgCl in 0.1(M) aqueous solution of AgNO3 will be the same as that in water.
Question 23. You are supplied with HCOOH (pKa =3.74), CH3COOH (pKa = 4.74), and NaOH solutions. To prepare a buffer solution of pH =3.8, which acid will you select? Give reason
Answer:
The buffer capacity of a buffer solution consisting of a weak acid and its salt becomes maximum when the pH ofthe buffer solution is equal to the pKa of the weak acid. Among the given acids, the pKa of HCOOH is very close to the desired pH of the buffer solution. Hence, one should use HCOOH for preparing the buffer.
Question 24. pH of a buffer solution composed of NH3 and NH4Cl is 9.26. Will there be any change in pH if 100 mL of distilled water is added to 100 mL of this buffer solution?
Answer:
For the buffer solution made up of NH3 and NH4Cl, the pH of the solutions is given by
⇒ \(p H=14-p K_b-\log \frac{\left[\mathrm{NH}_4 \mathrm{Cl}\right]}{\left[\mathrm{NH}_3\right]}\)
If 100 mL of distilled water is added to 100 mL of this buffer solution, no change occurs in the ratio of [NH4Cl] to [NH3] and the pH ofthe solution remains the same.
Class 11 Chemistry Chapter 7 Equilibrium
Question 25. Which of the given salts will undergo cationic or anionic or both cationic and anionic hydrolysis? NH4F, NaCN, AICl3, Na2CO3, NH4Cl
Answer:
Both NH4Cl and AlCl3 are the salts of strong acids and weak bases. In aqueous solution of such salts, cationic hydrolysis takes place. NaCN and Na2CO3 are the salts of weak acids and strong bases. In an aqueous solution of such salts, anionic hydrolysis takes place. NH4F is a salt of weak acid and weak base. In aqueous solution of such salt, both cationic and anionic hydrolysis take place.
Question 26. The values of pure water at 0°C and 25°C are x and y respectively. Is it greater than or less than y?
Answer:
For pure water \(p H=\frac{1}{2} p K_w\) Rise in temperature increases the value of Kw.
So, Kw(0°C) < Kw(25°C) & hence pKw(0°C) > pKw(100°C) as pKw = -log10. As in the case of pure water,
⇒ \(p H=\frac{1}{2} p K_w\) pH of pure at 0 °C will be greater than that at 25 °C. Hence, x > y.
Question 27. pKw = 12.26 at 100°C. What is the range of pH -scale at this temperature? What will be the pH of a neutral solution at this temperature?
Answer:
At 100 °C, pKw = 12.26. So, at this temperature, the pH scale ranges from 0 to 12.26. At this temperature,
⇒ \(\left[\mathrm{H}_3 \mathrm{O}^{+}\right]=\sqrt{K_w}\)
In a neutral aqueous solution.
Therefore, pH of this solution \(=\frac{1}{2} p K_w=\frac{1}{2} \times 12.26=6.13\)
Question 28. Why does the concentration of OH“ ions in pure water increase with temperature rise? Does this increase make pure water alkaline? Explain.
Answer:
In pure water, [OH–] = JKw. Kw increases with temperature, and so does [OH–]. This increase in [OH–] does not however mean that pure water becomes alkaline at a higher temperature as pure water always contains an equal number of H3O+ and OH– ions at any temperature.
Class 11 Chemistry Chapter 7 Equilibrium
Question 29. All Lewis bases are fact bases—explain. Each of HCO2 and HPO– can act both as Bronsted acid and hose—why? Write the formula of conjugate base and conjugate acid in each case.
Answer:
A chemical capable of accepting a proton is termed a Bronsted base, whereas a substance that can give a pair of electrons is referred to as a Lewis base. The NH3 molecule is capable of accepting a proton. Thus, it is a Brønsted base. The NH3 molecule provides a pair of electrons to establish a coordination bond with a proton. Thus, it also functions as a Lewis base. Consequently, it may be deduced that a Lewis base qualifies as a Bronsted base.
Question 30. Each of HCO3– and HPO– can act both as Bronsted acid and hose—why? Write the formula conjugate base and conjugate acid in each case.
Answer:
According to the Bronsted-Lowry concept, an acid is a proton donor and a base is a proton acceptor. Since the given species are capable of accepting and donating a proton, they can act as an acid as well as a base.
Question 31. Of the two solutions of acetic acid with concentrations 0.1(N) and 0.01(N), in which one does acetic acid have a higher degree of dissociation?
Answer:
Acetic acid is a weak acid. Such an acid undergoes partial ionization in water. The degree of ionization of a weak acid in its solution depends upon the concentration of the solution. The higher the concentration of a solution of a weak acid, the smaller the degree of ionization of the acid in that solution. So, the degree of ionization of acetic acid will be higher in 0.01(N) solution.
Question 32. At a certain temperature, the ionization constants of three weak acids HA, HB, and HC are 4.0 × 10-5 5.2 × 10-4, and 8.6 × 10-3, respectively. If the molar concentrations of their solutions are the same, then arrange them in order of their increasing strength.
Answer:
The larger the ionization constant (Ka) of an acid, the greater the extent to which it undergoes ionization in its aqueous solution, and hence the higher the concentration of H30+ ions produced by it in the solution. Alternatively, the larger the value of Ka of an acid, the greater its strength.
The increasing order ofthe given acids concerning their Ka: HA < HB < HC. Consequently, the order of the given acids in terms of increasing strength in their aqueous solutions will be HA < HB < HC.
Class 11 Chemistry Chapter 7 Equilibrium
Question 33. At 25°C, the ionization constant (Ka) of weak acid HA is 10-6. What will the value of the ionization constant (Kb) of its conjugate base (A-) be at that temperature?
Answer:
We know \(K_a \times K_b=10^{-14} \text { [at } 25^{\circ} \mathrm{C} \text { ]. } K_a \text { of } \mathrm{HA}=10^{-6} \text {. }\)
Therefore \(K_b \text { of } \mathrm{A}^{-}=\frac{10^{-14}}{K_a}=\frac{10^{-14}}{10^{-6}}=10^{-8} .\)
Question 34. Give an example of a salt solution whose pH is independent of salt concentration.
Answer:
In the case of a solution of a salt formed from a weak acid and a weak base, the pH of the solution does not depend upon the concentration of the salt. One such salt is CH3COONH4.
Question 35. For what kind of solids, is solid vapor equilibrium achieved easily?
Answer:
Solid substances that can be easily converted to vapors on heating under normal pressure (i.e., sublimable substances).
For example: Solid CO2, camphor, ammonium chloride, naphthalene, etc.
Question 36. At a fixed temperature, a liquid is in equilibrium with its vapors in a closed vessel. Which measurable tint quantity for the liquid gets fixed at equilibrium?
Answer:
When a liquid remains in equilibrium with its vapor in a closed vessel at a particular temperature, the vapor pressure of the liquid is found to acquire a fixed value.
Class 11 Chemistry Chapter 7 Equilibrium
Question 37. At 0°C and 1 atm pressure, why is the equilibrium established between water and ice regarded as dynamic?
Answer:
At 0°C and under 1 atm pressure, ‘water ice’ equilibrium is said to be dynamic since at equilibrium the rate of melting of ice is equal to the rate of freezing of water.
Question 38. Give two examples of chemical reactions for each of the following cases:
- Kp > Kc
- Kp<Kc
- Kp = Kc
Answer:
We know, Kp = Kc(RT)Δn
Kp will be greater than Kc if Δn > 0. Reactions for
Question 39. The following reaction is carried out in a closed vessel at a fixed temperature: A(g) 2B(g). The concentrations of A(g) and B (g) in the course ofthe reaction are as follows When does the reaction attain equilibrium? What are the equilibrium concentrations of A and B?
Answer:
From the time of 60 minutes, the concentrations of reactant and product are found to remain the same with time. Therefore, the reaction has arrived at an equilibrium state at 60 minutes.
At the state of equilibrium, [A] = 0.58 mol- L-1 and [B] = 0.84 mol- L-1
Question 40. Here are a few salts. Whose aqueous solution(s) at 25°C has (have) a pH greater than 7, less than 7, or equal to 7? (NH4)2SO4, CH3COONH4, K2CO3, NaNO3
Answer:
pH < 7 : (NH4)2SO4
pH> 7 : K2CO23
pH = 7 : CH3COONH4, NaNO3
MPBSE Solutions For Class 11 Chemistry Hydrogen
Class 11 Chemistry Hydrogen Question And Answers
Question 1. Name the isotopes of hydrogen and state their mass ratio.
Answer:
The three isotopes of hydrogen are:
Protium (H or H),
Deuterium (4H or D) and
Tritium (4H or T).
Their mass ratio is protium: deuterium: tritium =1:2:3.
Question 2. What is the source of solar energy?
Answer:
The main source of solar energy is the given nuclear fusion reaction: 4H —> 2He + 2+1e° (positron) + Energy
Question 3. Although Fe is placed above hydrogen in the electrochemical series, dihydrogen is not obtained by its reaction with nitric acid. Explain with reasons.
Answer:
HNO3 being a strong oxidizing agent oxidizes dihydrogen into the water and itself gets reduced to nitrogen dioxide;\(\mathrm{Fe}+6 \mathrm{HNO}_3 \rightarrow \mathrm{Fe}\left(\mathrm{NO}_3\right)_3+3 \mathrm{NO}_2+3 \mathrm{H}_2 \mathrm{O}\)
Question 4. Give an example and formula of a compound which on electrolysis liberates dihydrogen at the anode.
Answer:
Calcium hydride (CaH2) on electrolysis, liberates dihydrogen at the anode.
Solutions For Class 11 Chemistry Hydrogen
Question 5. How can one prepare H2 gas from water by using a reducing agent?
Answer:
Reaction between metals such as Na or. JC (strong reducing agents) and water produce hydrogen gas.
⇒ \(2 \mathrm{Na}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaOH}+\mathrm{H}_2 \uparrow\)

Question 6. Name two compounds, in one of which hydrogen is in +1 and in the other in -1 oxidation state.
Answer:
In HCl, hydrogen is in a +1 oxidation state and in NaH it is in a -1 oxidation state.
Question 7. Holli dihydrogen and carbon monoxide burn in the air with blue flame. How will you distinguish between them?
Answer:
Dihydrogen burns with a blue flame in the air to form water vapor which turns white anhydrous CuSO4 into hydrated copper sulfate (CuSO4-5H2O). However, carbon monoxide on combustion forms CO2 which does not bring about any change in CuSO4.
Question 8. What characteristics do you expect from an electron-deficient and an electron-rich hydride with respect to their structures?
Answer:
Electron-deficient hydrides function as electron acceptors, serving as Lewis acids, while electron-rich hydrides operate as electron donors, acting as Lewis bases. B2H6 functions as a Lewis acid, whereas NH3 acts as a Lewis base.
Question 9. Why the boiling point of HF is higher than that of other hydrogen halides?
Answer:
Due to the formation of strong intermolecular hydrogen bonding, the boiling point of HF is higher than that of other hydrogen halides.
Solutions For Class 11 Chemistry Hydrogen
Question 10. How can you separate H2 or D2 from He?
Answer:
Palladium, heated to a high temperature, is cooled in a helium environment mixed with hydrogen or deuterium. As a result, substantial quantities of H2 or D2 are absorbed by palladium, whereas He is not. Upon heating palladium, occluded H2 or D2 gels are released as free hydrogen or deuterium.
Question 11. Why ionic or salt-like hydrides are used to dry organic solvents?
Answer:
Ionic or salt-like hydrides are used to dry organic solvents because they readily react with water to form the corresponding metal hydroxide along with the evolution of H2O as- The solvent is then separated from the metallic hydroxide by distillation.
Question 12. Why concentration of D20 increase when electrolysis of water is carried out for a long period of time?
Answer:
Electrolysis of H20 occurs at a faster rate than D2O because the bond dissociation energy of the O—H bond is greater than that of the O—D bond. So, electrolysis of ordinary water for a prolonged period of time results in an increase in the concentration of D2O.
Question 13. How would you prepare deuterium peroxide (D2O2)?
Answer:
Deuterium peroxide (D2O2) can be prepared by the reaction between barium peroxide (BaO2) and deuterosulphuric acid (D2SO4).
⇒ \(\mathrm{BaO}_2+\mathrm{D}_2 \mathrm{SO}_4 \rightarrow \mathrm{BaSO}_4+\mathrm{D}_2 \mathrm{O}_2\)
Question 14. How will you prepare deuteroammonia (ND3) from N2?
Answer: Magnesium burns in nitrogen to produce magnesium nitride which further reacts with D2O to produce ND3 (deuteroammonia)
⇒ \(\begin{gathered} 3 \mathrm{Mg}+\mathrm{N}_2 \rightarrow \mathrm{Mg}_3 \mathrm{~N}_2 \\ \mathrm{Mg}_3 \mathrm{~N}_2+6 \mathrm{D}_2 \mathrm{O} \rightarrow 3 \mathrm{Mg}(\mathrm{OD})_2+2 \mathrm{ND}_3 \end{gathered}\)
Question 15. How will you prove that hypophosphorous acid (H3PO2) is a monobasic acid?
Answer:
When H3PO2 is treated with D2O, only one of its hydrogen atoms is replaced by D. So, it can be said that only one H-atom remains attached to O-atom in hypophosphorous acid (H3PO2). Therefore, it is a monobasic acid.
Solutions For Class 11 Chemistry Hydrogen
Question 16. Sodium chloride is less soluble in heavy water than ordinary water—why?
Answer:
As the dielectric constant of D2O is less than that of H2O, NaCl (sodium chloride) is less soluble in heavy water than ordinary water. +
Question 17. Explain why the water obtained after passing hard water through cation exchange resins is acidic.
Answer:
When hard water traverses an organic ion exchange resin, the resultant water is acidic due to the exchange of all metal ions in the water with H+ ions from the resin. Consequently, the resultant water is devoid of cations and possesses a high concentration of H+ ions. The water converts blue litmus paper to crimson.
Question 18. A sugar solution prepared in distilled water is passed successively through cation and anion exchange resins. What will be the taste of the collected water and why?
Answer:
Ion-exchange resins cannot remove sugar(non-electrolyte) from water. Therefore, when a sugar solution is passed successively through cation and anion exchange resins, after being collected tastes sweet.
Question 19. The hardness of the water in a tube well is 300 ppm. What do you mean by this statement?
Answer:
The statement means that in million parts by mass of the sample of water from the tube, well contains salts causing its hardness which are equivalent to 300 parts by mass of calcium carbonate.
Question 20. Will the water obtained by passing hard water through anion exchange resin, form lather with soap? Why?
Answer:
As the sample of water is not free from Ca2+ and Mg2+ ions, it will not form a lather with soap easily.
Question 21. A sample of water contains MgS04 and urea. How can they be eliminated easily?
Answer: They can be eliminated by a simple distillation method.
Solutions For Class 11 Chemistry Hydrogen
Question 22. It is better to preserve H202 in a polythene bottle than in a glass bottle—why?
Answer:
The decomposition of H2O2 is accelerated by the presence of glass, sunlight, and basic substances. So, H2O2 is preserved in polythene bottles rather than glass bottles.
Question 23. What do you understand by the expression ’30 volume H2O2 solution’?
Answer:
’30 volume H2O2 solution’ means that 1 mL of that solution yields 30 mL of oxygen at STP as a result of its complete decomposition.
Question 24. What do you mean by 20% H2O2 solution?
Answer:
20% H2O2 solution means that momT. of that solution contains 20g of H2O2.
Question 25. Calculate the percentage strength of 6.588 volume H2O2.
Answer:
Percentage strength of solution \(=\frac{\text { volume strength } \times 34}{11.2 \times 10}\)
\(=\frac{6.588 \times 34}{11.2 \times 10}=1.99\)MPBSE Solutions For Class 11 Chemistry Some P Block Elements
Class 11 Chemistry Some P Block Elements Long Questions And Answers
Question 1. Unlike diamond, graphite is a good conductor of electricity —explain.
Answer:
In a diamond crystal, each carbon atom is sp³-hybridized, meaning that all electrons in the valence shell of the carbon atom engage in the formation of sigma bonds with four adjacent carbon atoms.
- Consequently, there are no free electrons remaining in the lattice, rendering the diamond incapable of conducting electricity.
- Conversely, each carbon atom in graphite, being sp²-hybridized, utilizes three of its valence electrons to establish three σ-bonds with three adjacent carbon atoms.
- The fourth electron in the 2pz orbital, oriented perpendicularly to the carbon layer, establishes an n-bond through lateral overlap with the 2pz orbital of any of the three neighboring carbon atoms.
- The electrons of the π-bonds are delocalized throughout the entire crystal via resonance. Graphite exhibits excellent electrical conductivity owing to the existence of mobile electrons.
Read and Learn More Class 11 Chemistry
Question 2. Diamond is extremely hard but graphite is soft and slippery —explain with reason.
Answer:
In diamond crystal, each sp³-hybridized carbon atom is tetrahedrally bonded to four other carbon atoms via covalent connections.
- A multitude of tetrahedral units interconnected to create a three-dimensional extensive array of molecules with robust bonds extending in all directions. Due to its three-dimensional network of robust covalent connections, diamond exhibits exceptional hardness.
- Conversely, in graphite crystals, each sp²-hybridized carbon atom is bonded to three other carbon atoms, creating a network of planar hexagons.
- These two-dimensional layers are arranged in parallel planes, positioned horizontally above one another at a distance of 3.35 Å.
- Due to the weak van der Waals forces binding these layers, one layer can effortlessly glide over another with minimal pressure applied. This elucidates the reasons behind graphite’s softness and lubricity.
Class 11 Chemistry Some P Block Elements
Question 3. Carbon monoxide possesses both oxidising and reducing properties—why?
Answer:
The oxidation slate of carbon in carbon monoxide is +2. It stands in between its highest oxidation state (+4) and lowest oxidation state (-4). As a result, in chemical reactions, the carbon atom in CO may increase its oxidation number from +2 to +4 or it may decrease its oxidation number from +2 to -4. When the oxidation number increases, CO is oxidised and plays the role of a reducing agent. For example, at high temperatures, CO reduces black CuO to red metallic Cu.

On the other hand, in case of a decrease in the oxidation number of carbon, carbon monoxide undergoes reduction, i.e., it acts as an oxidising agent. For instance, in the following reaction, carbon monoxide oxidises hydrogen-producing water and itself gets reduced to methane

Question 4. How will you convert a mixture of CO and CO2 completely into
- CO2 and
- CO?
Answer:
When a mixture of CO2 and CO is passed over strongly heated CuO and kept in a combustion tube, CO2 remains unchanged while CO gets oxidised to CO2. As a consequence, only CO2 comes out of the combustion tube. In this way, the mixture is completely converted into CO2

On the other hand, when a mixture containing CO and CO2 is passed over white-hot coke, CO remains unchanged but CO2 gets reduced to CO. In this way, the mixture is completely converted into CO.

Class 11 Chemistry Some P Block Elements
Question 5. Mention 3 similarities like B and Si.
Answer:
Three similarities between boron and silicon are
1. Both boron and silicon react with caustic soda to form H2 gas.
2B + 6NaOH → + 3H2
Si + 2NaOH + H2O → Na2SiO3 + 2H2
2. Halides of both boron and silicon undergo hydrolysis to form weak acids
BCl3 + 3H2O→H3BO3+ 3HCl
SiCl4 + 4H2O→ H4SiO4 + 4HCl
3. Halides of both boron and silicon form complex compounds
BF3 +HF → HBF4; SiF4 + 2HF → H2SiF6
Question 6. When boron trichloride reacts with water, it only B3 forms [B(OH)4]– whereas aluminium trichloride forms [AI(H2O)4]3+ in acidified aqueous solution. State the hybridisation of boron and aluminium in these species and explain your answer
Answer:
Boron trichloride (BCl3 ) undergoes hydrolysis to form orthoboric acid [B(OH)3] at first As the atomic size of B is small and its electronegativity is high, B(OH)3 polarises HO molecule by accepting an OH– ion thereby forming [B(OH)4]– and releasing a proton.
BCl3 + 3H2O→B(OH)3 + 3HCl
B(OH)3 + H2O→[B(OH)4]–+ H+
There are no vacant d -orbitals in B as it lies in the second period and has only one s – and three p – orbitals. So, B can have four pairs of electrons in its valence shell, i.e., its maximum coordination number is 4.
For this reason, B(OH)3 accepts one OH” ion to form [B(OH)4]- in which B-atom is sp³ -hybridised. On the other hand, AlCl3 undergoes hydrolysis in acidic medium to form [Al(H2O)g]3+
AlCl3 + 6H2O →[Al(H2O)6]3+ + 3Cl(aq)–
In an acidic medium, the concentration of OH– ions is lower than H+ ions. Thus, Al3+ ions coordinate with H2O molecules and not with OH– ions. Due to the availability of vacant d -d-orbitals in Al3+ ions, it can expand its coordination number from 4 to 6. Thus, it can form the complex ion, [Al(H2O)6]3+ in which hybridisation state of Al is sp³d²
Class 11 Chemistry Some P Block Elements
Question 7. Give reasons for the following:
- Diamond-tipped tools are used for drilling and cutting purposes.
- Graphite is used as a lubricant.
- Silicones are water-repelling in nature.
- CO gets absorbed by ammoniacal cuprous chloride to form a complex but CO2 does not
Answer:
1. Diamond has the highest thermal conductivity among all known substances. Because of its high thermal conductivity, diamond-tipped tools do not overheat. Diamond is extremely hard as well. For these reasons, diamond-tipped tools are extensively used for drilling and cutting purposes.
2. Graphite has a layered structure in which any two successive layers are held together by weak van der Waals forces of attraction and thus one layer can smoothly slide over the other. For this reason, graphite acts as a lubricant.
3. Silicones are water-repelling in nature because they are surrounded by non-polar alkyl groups.
4. Carbon monoxide acts as a Lewis base due to the presence of a lone pair of electrons on carbon :C=0: and forms a soluble complex with ammoniacal cuprous chloride (CuCl).
CuCl + NH3 + CO→ [Cu(CO)NH3]+Cl–
On the other hand, carbon dioxide ![]() does not have a lone pair ofelectrons on carbon and cannot form complexes.
does not have a lone pair ofelectrons on carbon and cannot form complexes.
Question 8. What happens when
- A mixture of sand and sodium carbonate is melted on heating. 0 At 200°C and under high pressure, carbon monoxide is passed through caustic soda solution and the product is heated to 300°C.
- At high temperatures, metallic calcium is made to react with carbon and the product obtained is treated with water.
- Potassium ferrocyanide is heated in the presence of concentrated H2SO4 and the gas thus obtained is passed over finely divided nickel powder at 50°C.
- Silicon is heated with methyl chloride at high temperatures in the presence of copper.
- SiO2 is treated with HF.
Answer:
1. When a mixture of sand and sodium carbonate is melted by tremendous heat, sodium silicate and carbon dioxide are obtained.
SiO2 + Na2 CO3 → Na2SiO3 + CO2↑
2. When CO is passed through NaOH solution at 200°C under high pressure, sodium formate is produced.
NaOH + CO→ HCOONa
When sodium formate is heated at 300°C, sodium oxalate and hydrogen gas are formed.

3. Metallic calcium reacts with carbon at high temperatures to form calcium carbide. Calcium carbide on treatment with water produces acetylene and calcium hydroxide
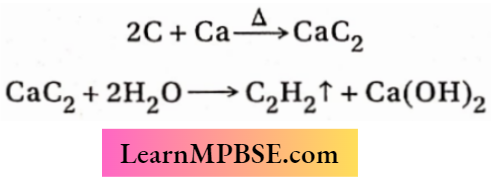
4. When potassium ferrocyanide is heated with concentrated sulphuric acid, potassium sulphate, ammonium sulphate, ferrous sulphate and carbon monoxide are formed. When the resulting CO gas is passed over finely divided nickel at 50°C, nickel tetracarbonyl (a coordinated complex) is formed
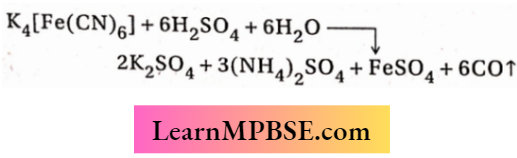
Ni + 4CO →(50°C) Ni(CO)4
5. When silicon is heated with methyl chloride at high temperature in the presence of copper, a mixture of mono-, di- and trimethylchlorosilane along with a small amount of tetramethylsilane is obtained
![]()
SiO2 reacts with HF to form silicon tetrafluoride. The initially formed SiF4 dissolves in HF to form hydrofluorosilicic acid.
SiO2 + 4HF→SiF4 + 2H2O ; SiF4 + 2HF → H2SiF6
Class 11 Chemistry Some P Block Elements
Question 9. Starting from boric acid how can you prepare—
- Boric anhydride
- Boron trichloride
- Boron trifluoride
- Meta and tetraboric acid
- Boron hydride
- Ethyl borate
Answer:
1. Boric anhydride is prepared by heating boric acid at high temperatures

2. Boric acid is first converted to boric anhydride, which on heating with Cl2 forms boron trichloride.
B2O3 + 3C + 3Cl2→2BCl3 + 3CO
3. Boric acid, on heating with CaF2 and cone. H2S04 forms boron trifluoride.
CaF2 + H2SO4→CaSO4 + H2F2
2H3BO3 + 3H2F2 → 2BF3 + 6H2O
4. Orthoboric acid on heating at 100°C and 160°C forms metaboric acid and tetraboric acid respectively.
Metaboric Acid:

Tetraboric Acid:

5. Boric acid is first converted to boric anhydride which on heating with Mg-powder forms magnesium boride. On reacting dilute HCl with magnesium boride, boron hydrides are formed.

Ethyl borate is formed by heating a mixture of boric acid and ethanol in the presence of concentrated H2SO4.
3C2H5OH + H3BO3 → (C2H5)3BO3 + 3H2O
Class 11 Chemistry Some P Block Elements
Question 10. Consider the compounds, BCI3 and CCl4. How will they behave with water? Justify.
Answer:
BCl3 is an electron-deficient molecule because the core boron atom possesses only six electrons in its valence shell. Consequently, it can take a pair of electrons provided by water and undergo hydrolysis to produce boric acid (H3BO3) and HCl.
The central atom of the molecule, designated as atom B, possesses six valence electrons. Shell
Conversely, the carbon atom in CCl4 possesses 8 electrons in its valence shell and lacks unoccupied d-orbitals to expand its octet. CCl4 cannot take a pair of electrons from H2O; therefore, it does not undergo hydrolysis.
MPBSE Class 11 Chemistry Solutions For Equilibrium
Question 1. HPO42- can act both as a Bronsted base and as a Bronsted acid. Write the equation of equilibrium established by HPO42- as an acid and a base in an aqueous solution. Also, write the expressions of Ka & Kb in two cases. What are the conjugate acid and base of HS–?
Answer:
HPO42- can denate and accept protons in aqueous solution. Thus it can serve both as an acid and base.
⇒ \(\begin{aligned}
& \mathrm{HPO}_4^{2-}(a q)[\text { Acid }]+\mathrm{H}_2 \mathrm{O}(l) \rightleftharpoons \mathrm{PO}_4^{3-}(a q)+\mathrm{H}_3 \mathrm{O}_2^{+}(a q) \\
& \mathrm{HPO}_4^{2-}(a q)[\text { Base }]+\mathrm{H}_2 \mathrm{O}(l) \rightleftharpoons \mathrm{H}_2 \mathrm{PO}_4^{-}(a q)+\mathrm{OH}^{-}(a q)
\end{aligned}\)
⇒ \(K_a=\frac{\left[\mathrm{PO}_4^{3-}\right] \times\left[\mathrm{H}_3 \mathrm{O}^{+}\right]}{\left[\mathrm{HPO}_4^{2-}\right]} ; K_b=\frac{\left[\mathrm{H}_2 \mathrm{PO}_4^{-}\right] \times\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{HPO}_4^{2-}\right]}\)
HS– (aq) + H2O(l) ⇌ H2S(aq) + OH– (aq)
Hence, the conjugate acid of HS– is H2S.
HS–(aq) + H2O(l) ⇌ S2-(aq) + H3O+(aq)
Therefore, the conjugate base of HS– is S2--.
Read and Learn More Class 11 Chemistry
Question 2. An aqueous solution of sodium bisulfate is acidic, whereas an aqueous solution of sodium bicarbonate is basic—Explain.
Answer:
Since sodium bisulfate (NaHSO4) is a salt of strong acid (H2SO4) and strong base (NaOH), it is not hydrolyzed in an aqueous solution. NaHS04 in its solution dissociates completely to form Na+ and HSO4 ions and HSO4 ions so formed get ionized to form H3O+ and SO2-4 ions. As a result, the aqueous solution of NaHSO4 becomes acidic.
⇒ \(\begin{gathered}
\mathrm{NaHSO}_4(a q) \rightarrow \mathrm{Na}^{+}(a q)+\mathrm{HSO}_4^{-}(a q) \\
\mathrm{HSO}_4^{-}(a q)+\mathrm{H}_2 \mathrm{O}(l) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(a q)+\mathrm{SO}_4^{2-}(a q)
\end{gathered}\)
NaHCO3 in its solution dissociates completely to form Na+ and HCO3 ions [NaHCO3(aq)-Na+(aq) + HCO3 (aq) ]. HCO3 can act both as an acid and a base in aqueous solution.
⇒ \(\begin{aligned}
& \mathrm{HCO}_3^{-}(a q)[\text { Acid }]+\mathrm{H}_2 \mathrm{O}(l) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(a q)+\mathrm{CO}_3^{2-}(a q) \\
& \mathrm{HCO}_3^{-}(a q)[\text { Base }]+\mathrm{H}_2 \mathrm{O}(l) \rightleftharpoons \mathrm{H}_2 \mathrm{CO}_3(a q)+\mathrm{OH}^{-}(a q)
\end{aligned}\)
Class 11 Chemistry Solutions For Equilibrium
Question 3. Calculate the formation constant of [Ag(NH3)2]+
Answer:
⇒ \(\mathrm{Ag}^{+}(a q)+\mathrm{NH}_3(a q) \rightleftharpoons\left[\mathrm{Ag}\left(\mathrm{NH}_3\right)\right]^{+} ; K_1=3.5 \times 10^3\)
⇒ \(\begin{aligned}
{\left[\mathrm{Ag}\left(\mathrm{NH}_3\right)\right]^{+}+\mathrm{NH}_3(a q) \rightleftharpoons\left[\mathrm{Ag}\left(\mathrm{NH}_3\right)_2\right]^{+} ; } \\
K_1=1.7 \times 10^3 \ldots
\end{aligned}\)
Equation (3) represents the formation reaction of [Ag(NH3)2]2+. Therefore, the equilibrium constant for the reaction represented by equation (3) will be the formation constant for [Ag(NH3)2]2+. As equation (3) is obtained by adding equations (1) and (2), the formation constant (fcy) for [Ag(NH3)2]2+ equals ky x k2.
Thus, ky = ky X k2 = (3.5 × 103) × (1.7 × 103) = 5.95 × 106
Question 4. The first and second dissociation constants of an acid H2A are 1 × 10-5 and 5 × 10-10 respectively. Calculate the value of the overall dissociation constant.
Answer:
⇒ \(\begin{aligned}
\mathrm{H}_2 \mathrm{~A}(a q)+\mathrm{H}_2 \mathrm{O}(l) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(a q)+\mathrm{HA}^{-}(a q) ; \\
K_1=1 \times 10^{-5} \ldots(1)
\end{aligned}\)
⇒ \(\begin{array}{r}
\mathrm{HA}^{-}(a q)+\mathrm{H}_2 \mathrm{O}(l) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(a q)+\mathrm{A}^{2-}(a q) ; \\
K_2=5 \times 10^{-10} \ldots
\end{array}\)
The overall dissociation reaction is the sum of the. reactions (1) and (2). H2A(aq) + 2H2O(Z) 2H30+(aq) + A3-(aq) Thus, the overall disputation constant K = K1 × K2 = 1 × 10-5 and 5 × 10-10= 5 ×10-15
Question 5. a -D-glucose Beta -D glucose, the equilibrium constant for this is 1.8. Calculate the percentage of a -D glucose at equilibrium.
Answer:
Suppose, the initial concentration of a -D glucose is a mol L-1 and its degree of conversion to p -D glucose at equilibrium is x mol-L-1
Class 11 Chemistry Solutions For Equilibrium
Therefore, the concentration of a -D glucose and D glucose at equilibrium will be as follows—
⇒ \(\begin{array}{ccc}
\begin{array}{c}
\text { Initial concentration } \\
\left(\text { in mol } \cdot \mathrm{L}^{-1}\right. \text { ) }
\end{array} & a & 0 \\
\begin{array}{c}
\text { Equilibrium concentration } \\
\left(\text { in mol } \cdot \mathrm{L}^{-1}\right. \text { ) }
\end{array} & a-a x & a x
\end{array}\)
Equilibrium constant for this process, \(K=\frac{[\beta-\mathrm{D}-\text { glucose }]}{[\alpha-\mathrm{D}-\text { glucose }]}\)
⇒ \(1.8=\frac{a x}{a(1-x)}=\frac{x}{1-x}\) or, \(x=\frac{1.8}{2.8}=0.6428\)
Thus, the percentage of a -D-glucose at equilibrium is \(\frac{a(1-0.6428)}{a} \times 100=35.72 \%\)
Question 6. Solid Ba(NO3)2 is gradually dissolved in a 1.0 x 10-4 M Na2CO3 solution. At what concentration of Ba2+ will a precipitate begin to form? ( Ksp for BaCO3 = 5.1 × 10-9 )
Answer:
Ba(NO3)2 reacts with Na2CO3 to form BaCO3. BaCO3 is a sparingly soluble compound that forms the following equilibrium in its saturated solution.
⇒ \(\mathrm{BaCO}_3(s) \rightleftharpoons \mathrm{Ba}^{2+}(a q)+\mathrm{CO}^{2-}(a q)\)
For \(\mathrm{BaCO}_3, K_{s p}=\left[\mathrm{Ba}^{2+}\right]\left[\mathrm{CO}_3^{2-}\right]\)
In the solution \(\left[\mathrm{CO}_3^{2-}\right]=1 \times 10^{-4} \mathrm{M}\)
BaCO3 will precipitate when [Ba2+][CO2–] → Ksp, i.e., when
⇒ \(\left[\mathrm{Ba}^{2+}\right]\left[\mathrm{CO}_3^{2-}\right]>5.1 \times 10^{-9}\left(\text { as } K_{s p}\left[\mathrm{BaCO}_3\right]=5.1 \times 10^{-9}\right)\) \(\text { If }\left[\mathrm{CO}_3^{2-}\right]=1 \times 10^{-4} \mathrm{M} \text {, then }\left[\mathrm{Ba}^{2+}\right]>\frac{5.1 \times 10^{-9}}{1 \times 10^{-4}}\)
Or, [Ba2+] > 5.1 × 10-5M
Thus, the precipitation of BaCO3 will start when [Ba2+] in the solution is grater than 5.1 × 10-5 M.
Class 11 Chemistry Solutions For Equilibrium
Question 7. 2.5 m l of \(\frac{2}{5} \mathrm{M}\) weak monoacdic base Kb = 1 x 10-125at 25°C ) is titrated with \(\frac{2}{15}\) in water at 25°C. Calculate the concentration of H + at the equivalence point. (kw = 1 × 10-14)
Answer:
Ana. 2.5 mL of \(\frac{2}{15}\) M monobasic acid \(\equiv \frac{2}{5} \times 2.5 \equiv 1\) mmol of the base.
Suppose, VmL of \(\frac{2}{15} \mathrm{M}\) HC1 is required for the neutralisation. \(V \mathrm{~mL} \text { of } \frac{2}{15} \mathrm{M} \mathrm{HCl} \equiv \frac{2}{15} \times V \mathrm{mmol} \mathrm{HCl} .\)
Therefore \(\) mmol HCL will neutralise 1mmol of the base Hence \(\frac{2 \times V}{15}=1 \text { or, } V=7.5 \mathrm{~mL}\)
The total volume of the solution after neutralization = (2.5 + 7.5) mL = 10 mL.
The number of mmol of the salt formed in the neutralization =1 mmol.
So, the concentration of the salt in the final solution \((C)=\frac{1}{10}=0.1 \mathrm{M}\) As the resulting salt is formed from a weak base and a strong acid, it undergoes hydrolysis. The pH of the solution of such a salt is given by the pH
\(=7-\frac{1}{2} p K_b-\frac{1}{2} \log C\) \(\text { As } K_b=10^{-12}, p K_b=-\log _{10}\left(10^{-12}\right)=12\)
Therefore \(p H=7-\frac{1}{2} \times 12-\frac{1}{2} \log (0.1)=7-6+0.5=1.5\) and the concentration of H+(aq) ions at the equivalence point is [H+]
= 10-PH M = 10-1-5 M = 0.316 M.