MPBSE Class 11 Chemistry Nascent Hydrogen
Hydrogen at the moment of its formation is called nascent hydrogen. The reducing property of nascent hydrogen is much greater than that of ordinary molecular hydrogen.
Nascent Hydrogen Example:
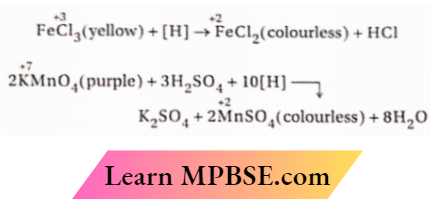
Ordinary’ molecular hydrogen cannot reduce acidic potassium permanganate or ferric chloride solution. However, when zinc dust is added to these solutions, the solutions become colorless and this is because, nascent hydrogen liberated in situ by the action of zinc on dilute sulphuric acid brings about the reduction of these compounds, discharging the colors of these solutions.
⇒ \(\mathrm{Zn}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{ZnSO}_4+2[\mathrm{H}](\text { nascent hydrogen })\)
Nascent hydrogen is a hydrogen atom released during a reaction, and it reacts with something else very, very rapidly. Now, there isn’t any physical difference, both are atoms, but based on the surrounding conditions, if the atom is stable, it is called atomic. If not, it is called nascent.
MPBSE Class 11 Chemistry Identification And Uses Of Dihydrogen
Identification of dihydrogen:
- The gas burns in the air with a pale blue flame and produces water. That the product is water is identified by the fact that it turns white anhydrous CuSO, blue.
- The gas is completely adsorbed by spongy palladium metal at ordinary temperature and die adsorbed gas is set free on heating the hydrogenised palladium to dull-red.
| Class 10 Science | Class 11 Chemistry |
| Class 11 Chemistry | Transformation of Sentences |
| Class 8 Maths | Class 8 Science |
Uses of dihydrogen:
- Dihydrogen is largely used in the synthesis of ammonia which is a starting material for the manufacture of nitric acid and various fertilizers such as urea, ammonium sulfate, etc.
- Large quantities of dihydrogen are consumed in the vanaspati industry for the conversion of vegetable oil into vanaspati ghee (hardening of oils).
- It is used in the manufacture of bulk chemicals such as methanol.\(\mathrm{CO}(g)+2 \mathrm{H}_2(g) \underset{\mathrm{Co}+\text { catalyst }}{\stackrel{700 \mathrm{~K} / 200 \mathrm{~atm}}{\longrightarrow}} \mathrm{CH}_3 \mathrm{OH}(l)\)
- It is used in the manufacture of metal hydrides.
- In metallurgy, dihydrogen is used to reduce heavy metal oxides to metals.
- It is used in the preparation of many useful chemicals such as hydrochloric acid.
- It is used in tire atomic hydrogen torch (produces temperature ~ 4000°C ) and oxy-hydrogen torch (produces temperature between 2270-2770°C ) for cutting and welding purposes.
- Liquid dihydrogen mixed with liquid oxygen is used as a rocket fuel in space research. It is also used in fuel cells for generating electrical energy.
- It is used in the manufacture of synthetic petrol.
- With helium, it is used for filling balloons employed for atmospheric study.
MPBSE Class 11 Chemistry Dihydrogen As A Fuel Hydrogen Economy
The production of energy in our present-day life involves mainly the use of fossil fuels such as coal, petroleum, natural gas, etc.
- In order to meet the growing demand for energy, the world’s fossil fuel reserves are getting depleted at an alarming rate and possibly they may get exhausted by the middle of the 21st century.
- In fact, the rate of consumption of fossil fuels is very much faster than the rate of their renewal in nature and it is the basic reason for their shortage. Therefore, new sources of energy are to be found.
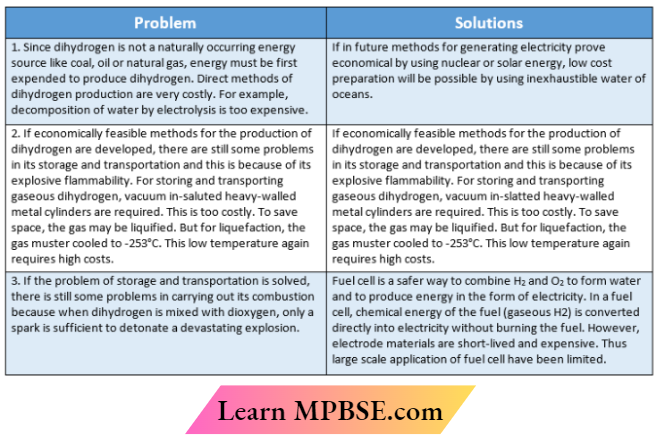
- Dihydrogen has been considered as an alternative source of energy. The basic principle of a hydrogen economy is the storage and transportation of energy in the form of liquid or gaseous dihydrogen.
MPBSE Class 11 Chemistry Advantages of using dihydrogen as a fuel:
- The major advantage of using dihydrogen as a fuel is that it provides a pollution-free atmosphere because its combustion product is only water.
- The heat of combustion per gram of dihydrogen is higher than any other fuel. For example, the amount of energy obtained from dihydrogen is 142 kJ. g-1 while those obtained from gasoline, coal, paper, and wood are 48, 29.3, 20, and 15 kJ . g-1 respectively.
- It is abundant in a combined state as water.
- An automobile engine using dihydrogen is about 25- 50% more efficient than an automobile engine using gasoline.
- The time required for the regeneration of dihydrogen from its combustion product water is much shorter than that of fossil fuel from its combustion product CO2.
- Although dihydrogen appears to be a very good future fuel, there are some typical problems that are to be solved before we adopt the hydrogen economy.
MPBSE Class 11 Chemistry Hydrides
Dlhydrogcn, under appropriate reaction conditions, combines with almost all elements, except noble gases, to form binary compounds called hydrides. The hydrides may be represented by the general formula EHx (Example, MgH2) or EmHn (ExampleB2H6 ), where E is a symbol of the element.
Nascent Hydrogen Class 11 Notes
All the main group elements except noble gases and probably indium and thallium, all lanthanides, all actinides, and transition metals such as Sc, Y, La, Ac, Tc, Zr, and Hf (to a lesser extent V, Nb, Tb, Cr, Cu, and Zn) form hydrides.
Depending upon the behavior and the nature of bonding, the hydrides can be classified into three main categories:
- Ionic or saline or salt-like hydrides;
- Covalent or molecular hydrides and
- Metallic or interstitial hydrides
MPBSE Class 11 Chemistry Ionic Or Salt-Like Hydrides
These hydrides are formed when hydrogen combines with electropositive alkali metals or alkaline earth metals of s -s-block having electronegativity less than hydrogen (2.1). The formation of ionic hydrides involves the transfer of one electron from the metal atom to the H-atom. Some common examples are LiH, NaH, CaH2, SrH2, etc. The hydride ion (H ) with [He] configuration is soft enough to be strongly polarised by the small Be2+ and Mg2+ ions and for this reason, BeH2 and MgH2 are significantly covalent. In fact, they exist in polymeric form.
Ionic Or Salt-Like Hydrides Preparation: These hydrides are prepared by heating the metal in an atmosphere of hydrogen under pressure over the temperature range of 150-600°C. For example:
⇒ \(2 \mathrm{Li}+\mathrm{H}_2 \stackrel{600^{\circ} \mathrm{C}}{\longrightarrow} 2 \mathrm{LiH}\)
⇒ \(2 \mathrm{M}+\mathrm{H}_2 \stackrel{400^{\circ} \mathrm{C}}{\longrightarrow} 2 \mathrm{MH}[\mathrm{M}=\mathrm{Na}, \mathrm{K}, \mathrm{Rb}, \mathrm{Cs}]\)
⇒ \(\mathrm{M}^{\prime}+\mathrm{H}_2 \stackrel{150-300^{\circ} \mathrm{C}}{\longrightarrow} \mathrm{M}^{\prime} \mathrm{H}_2\left[\mathrm{M}^{\prime}=\mathrm{Ca}, \mathrm{Ba}, \mathrm{Sr}\right]\)
Nascent Hydrogen Definition Class 11
Ionic Or Salt-Like Hydrides Physical properties:
- Ionic hydrides are non-volatile, non-conducting white crystalline solids.
- They have high melting and boiling points.
- They- have high density, generally higher than that of the metals from which they are formed. This is due to the fact that H“ ions occupy holes in the lattice of the metal, without distorting the metal lattice.
- They have high values of heat of formation\(\left(\Delta H_f^0\right)\)
- Their thermal stability decreases with increasing the cationic size of the metal down the group in the periodic table: LiH > NaH > KH > RbH > CsH
- In the molten state, they conduct electricity with the liberation of dihydrogen at the anode. This confirms the presence of hydride ions (H–) in them. For example:
⇒ \(\mathrm{CaH}_2(\text { molten }) \rightarrow \mathrm{Ca}^{2+}+2 \mathrm{H}^{-}\)
Cathode: \(\mathrm{Ca}^{2+}+2 e \rightarrow \mathrm{Ca}\)
Anode: \(2 \mathrm{H}^{-} \rightarrow \mathrm{H}_2+2 e\)
MPBSE Class 11 Chemistry Ionic Or Salt-Like Hydrides Chemical Properties
1. They react vigorously with water and other protic solvents such as ethanol, ammonia, etc., to liberate dihydrogen.
⇒ \(\mathrm{NaH}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{NaOH}+\mathrm{H}_2 \uparrow\)
⇒ \(\mathrm{LiH}+\mathrm{CH}_3 \mathrm{OH} \rightarrow \mathrm{LiOCH}_3+\mathrm{H}_2 \uparrow\)
⇒ \(\mathrm{NaH}+\mathrm{NH}_3 \rightarrow \mathrm{NaNH}_2+\mathrm{H}_2 \uparrow\)
2. Except LiH, they bum in the air on strong heating (400°-500°C) due to their decomposition into hydrogen which is inflammable.
3. There are strong reducing agents (especially under heated conditions). For example:
⇒ \(\mathrm{LiH}+\mathrm{CO}_2 \rightarrow \mathrm{HCOOLi} \text { [lithium formate] }\)
⇒ \(\mathrm{NaH}+2 \mathrm{CO} \rightarrow \mathrm{HCOONa} \text { [sodium formate] }+\mathrm{C}\)
Mpbse Class 11 Chemistry Notes Pdf
⇒ \(\mathrm{PbSO}_4+2 \mathrm{CaH}_2 \rightarrow \mathrm{PbS}+2 \mathrm{Ca}(\mathrm{OH})_2\)
⇒ \(6 \mathrm{LiH}+\mathrm{N}_2 \rightarrow 2 \mathrm{Li}_3 \mathrm{~N}+3 \mathrm{H}_2 \uparrow ; 3 \mathrm{CaH}_2+\mathrm{N}_2 \rightarrow \mathrm{Ca}_3 \mathrm{~N}_2+3 \mathrm{H}_2 \uparrow\)
MPBSE Class 11 Chemistry Jonic hydrides Uses:
Jonic hydrides are used as
- A ready source of di¬ hydrogen,
- Solid fuel,
- Reducing agents and
- To prepare the very important reducing agents like lithium aluminum hydride (liajh4) & sodium borohydride (nabh4).
Covalent Or Molecular Hydrides
- When hydrogen combines with elements of comparatively high electronegativity such as p -p-block elements, covalent or molecular hydrides are formed. The common elements forming molecular hydrides are B, C, N, O, F, Si, P, S, Cl, Ga, Ge, As, Se, Br, In, Sn, Pb, etc.
- The two s -s-block elements namely Be and Mg also form this type of hydride. The formation of covalent hydride is primarily due to the fact that the electronegativity difference between these elements and hydrogen is not much higher.
- Their general formula is XHn (for s -block elements) or XH8-n (for p -block elements) where n is the number of valence electrons of the element. These hydrides usually exist as discrete covalent molecules which are held together by weak van der Waals forces of attraction and hence are called covalent or molecular hydrides.
Molecular Hydrides Classification: Covalent or molecular hydrides can be further classified into three categories according to their relative number of electrons and bonds present in their Lewis structures.
1. Electron-deficient hydrides: These hydrides have less number of electrons in the valence shell of the central atom (usually, less than eight electrons in the valance shell). So their monomers do not satisfy the usual octet rule.
The elements of group-13 form this type of hydride, which gains stability through the formation of dimers (Example., B2H6, Ga2H6, etc.) and polymers [Example (AlH3)w, etc.]. Due to a deficiency of electrons, these hydrides act as Lewis acids and form complex entities with Lewis bases such as \(\ddot{\mathrm{N}}_3\) H– ion, etc.
Hydrogen Chapter Class 11 Short Notes
⇒ \(\mathrm{B}_2 \mathrm{H}_6+2 \mathrm{LiH} \rightarrow 2 \mathrm{Li}^{+}\left[\mathrm{BH}_4\right]^{-} \text {[Lithium borohydride] }\)
2. Electron-precise hydrides: These hydrides have an exact number of electrons in the valence shell of the central atom so as to write their conventional Lewis structures. Examples are hydrides of the elements of the group- 14 such as CH4, SiH4, GeH4, etc. They are tetrahedral in structure.
3. Electron-rich hydrides: These hydrides have more electrons in the valence shell of the central atom so as to write their conventional Lewis structures. The excess electrons are present in the form of lone pairs. Examples are hydrides of elements of group-15 to 17 such as \(\ddot{\mathrm{N}_H}{ }_3, \mathrm{H}_2 \ddot{\mathrm{O}}:, \mathrm{H} \ddot{\mathrm{i}} \mathrm{i}:, \mathrm{H}_2 \ddot{\mathrm{s}}:\) etc. Due to the presence of lone pairs of electrons, these hydrides act as Lewis bases and form complex entities with Lewis acids such as BF3, B2H6, Ga2 H,6, etc.
⇒ \(\mathrm{BF}_3+\ddot{\mathrm{NH}}_3 \rightarrow\left[\mathrm{BF}_3 \leftarrow: \mathrm{NH}_3\right]\)
⇒ \(\mathrm{B}_2 \mathrm{H}_6+2 \ddot{\mathrm{NH}}_3 \rightarrow\left[\mathrm{BH}_2\left(\mathrm{NH}_3\right)_2\right]^{+}\left[\mathrm{BH}_4^{-}\right]\)
Nomenclature : The systematic names of the molecular hydrides are usually derived from the name of the element by attaching the suffix ‘ane! For example chlorane for HCl, phosphane for PH3, oxidane for H2O, azane for NH3, sulphane for H2S, etc. However, common names like hydrogen chloride, phosphine, water, ammonia, and hydrogen sulfide are more commonly used.
MPBSE Class 11 Chemistry Nomenclature Preparation:
1. These hydrides can be obtained by direct combination of the elements at high temperatures.
⇒ \(\mathrm{N}_2+3 \mathrm{H}_2 \stackrel{\Delta}{\longrightarrow} 2 \mathrm{NH}_3 ; \mathrm{O}_2+2 \mathrm{H}_2 \stackrel{\Delta}{\longrightarrow} 2 \mathrm{H}_2 \mathrm{O}\)
2. These may be obtained by the hydrolysis of compounds such as borides, silicides, phosphides, sulfides, carbides, etc. For example:
⇒ \(\mathrm{Mg}_3 \mathrm{~B}_2+6 \mathrm{HCl} \rightarrow 3 \mathrm{MgCl}_2+\mathrm{B}_2 \mathrm{H}_6\)
⇒ \(\mathrm{Mg}_2 \mathrm{Si}+4 \mathrm{HCl} \rightarrow 2\mathrm{MgCl}_2+\mathrm{SiH}_4\)
⇒ \(\mathrm{Al}_4 \mathrm{C}_3+12 \mathrm{H}_2 \mathrm{O} \rightarrow 4 \mathrm{Al}(\mathrm{OH})_3+3 \mathrm{CH}_4\)
⇒ \(\mathrm{Mg}_3 \mathrm{~N}_2+6 \mathrm{H}_2 \mathrm{O} \rightarrow 3 \mathrm{Mg}(\mathrm{OH})_2+2 \mathrm{NH}_3\)
Hydrogen Chapter Class 11 Short Notes
⇒ \(\mathrm{FeS}+2 \mathrm{HCl} \rightarrow \mathrm{FeCl}_2+\mathrm{H}_2 \mathrm{~S}\)
⇒ \(\mathrm{Ca}_3 \mathrm{P}_2+6 \mathrm{H}_2 \mathrm{O} \rightarrow 3 \mathrm{Ca}(\mathrm{OH})_2+2 \mathrm{PH}_3\)
3. These may also be prepared by the reduction of the appropriate anhydrous chloride by LiAlH4. For example:
⇒ \(4 \mathrm{BCl}_3+3 \mathrm{LiAlH}_4 \rightarrow 2 \mathrm{~B}_2 \mathrm{H}_6+3 \mathrm{LiCl}+3 \mathrm{AlCl}_3\)
⇒ \(\mathrm{MCl}_4+\mathrm{LiAlH}_4 \rightarrow \mathrm{LiCl}+\mathrm{AlCl}_3+\mathrm{MH}_4[\mathrm{M}=\mathrm{Si}, \mathrm{Ge}, \mathrm{Sn}]\)
MPBSE Class 11 Chemistry Nomenclature Physical Properties:
- These hydrides consist of discrete covalent molecules held together by relatively weaker van der Waals forces and dipole-dipole interaction and in some cases by hydrogen bonding. Hence, these axe gases, liquids, or solids of low melting and boiling points.
- The hydrides of the first element of groups 15, 16, and 17 (i.e.„ NH3, H2O, and HF ) have abnormally high boiling points as compared to the hydrides of other elements of each group. The highly electronegative central atoms of these compounds strongly polarise the covalent bond with H and as a result, they remain associated through intermolecular hydrogen bonding. Due to such associations, they have abnormally high boiling points.
- They are bad conductors of electricity.
- Being covalent in nature, they are much soluble in organic solvents.
MPBSE Class 11 Chemistry Nomenclature Chemical Properties
1. Since the electronegativity of the central atoms increases from left to right along a period of the periodic table, covalent hydrides become progressively more acidic. For example, NH3 (basic), H2O (amphoteric), and HF (acidic).
2. The electron-rich hydrides behave as Lewis bases (electron donors), while the electron-deficient hydrides behave as Lewis acids (electron acceptors). They react with each other to form complex compounds.
⇒ \(\mathrm{B}_2 \mathrm{H}_6+2 \ddot{\mathrm{NH}}_3 \longrightarrow 2\left[\mathrm{BH}_3 \leftarrow: \mathrm{NH}_3\right]\)
Nascent Hydrogen Class 11 Notes
3. They undergo decomposition to their respective elements when heated.
⇒ \(\mathrm{SiH}_4 \stackrel{>300^{\circ} \mathrm{C}}{\longrightarrow} \mathrm{Si}+2 \mathrm{H}_2\)
The thermal stability in a group decreases as the electronegativity decreases with an increase in the size of the central atom down the group. For example, the thermal stability of group-15 hydrides follows the order:
NH3 > PH3 > ASH3 > SbH3 > BiH3
MPBSE Class 11 Chemistry Metallic Or Interstitial Hydrides
- These hydrides are binary compounds of hydrogen and transition elements and are generally formed when d -d-block elements of group-3, 4, 5 (Sc, Ti, V, Zr, Nb, La, Hf, Ta, Ac, etc.), 6 (only Cr), 10, 11, 12, (Pb, Cu, Zn, etc.) and /-block elements (Ce, Eu, Yb, Th, U, etc.) are subjected to react with dihydrogen at elevated temperatures.
- The metals of group-7, 8, and 9 do not form hydrides, and the region of the periodic table from group- 7 to 9 is called hydride gap. These hydrides exhibit properties similar to those of the parent metal and hence are called metallic hydrides.
- In these hydrides, hydrogen atoms being small in size occupy the interstitial space of the metal lattice producing distortion without any change of its type. That’s why, these hydrides are called interstitial hydrides. This distortion of the lattice makes the hydrides brittle.
- However, it has been known from recent studies that except the hydrides of Ni, Pb, Ce, and Ac, other hydrides of this class have lattices different from those of the parent metal. These hydrides may also be regarded either as alloys or as solid solutions of hydrogen in metal.
- The composition of hydrides of some lanthanides and actinides belonging to f-block may not correspond to a simple whole number ratio and therefore, they are called non-stoichiometric hydrides. Their composition has been found to vary with temperature and pressure. LaH2.87’ YbH2.55’ ZrH1.3-1.75’ VH0.56’ NiH0.6-0.7’ PdH0.6-0.8 etc.
MPBSE Class 11 Chemistry Metallic Or Interstitial Hydrides Properties
- These hydrides are hard and conduct heat and electricity.
- They have a metallic luster and magnetic properties.
- They generally undergo reversible decor position into dihydrogen and metal and therefore, they are strong-reducing agents.
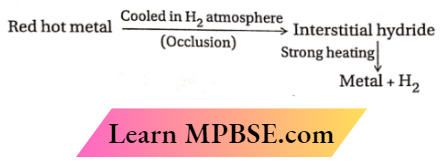
Occlusion of dihydrogen:
- Some transition metals can adsorb large volumes of dihydrogen on their surface due to the formation of interstitial hydrides. For example, when red-hot Pd is cooled in the atmosphere of dihydrogen, it adsorbs 935 times its own volume of dihydrogen.
- This property of adsorption of a gas by a metal is known as occlusion. The amount of dihydrogen occluded depends upon the nature and the physical state of the metal involved.
- For example, the occlusion power decreases in the order of colloidal Pd > Pt > Au >Ni. Due to the occlusion of H2, the metal lattice becomes expanded and unstable. For this reason, the occluded dihydrogen is liberated on strong heating.
Chemistry Nascent Hydrogen Notes
MPBSE Class 11 Chemistry Occlusion of dihydrogen Uses
- Metal hydrides formed as a result of the occlusion of H2 gas can be used as a hydrogen storage media (i.e., as a source of energy).
- Occlusion can be used to remove impurities like N2 present in H2.
- Occlusion can also be used to separate H2 from He.
- Metals like Ni, Pd, Pt, etc. which can adsorb large volumes of dihydrogen are widely used in catalytic hydrogenation (reduction) reactions for preparing a large number of compounds.
- Besides the three categories of hydrides mentioned above, elements having electronegativity between 1.4 to 2.0 form some polymeric hydrides in which the monomer molecules are held together in two or three dimensions by hydrogen bridges. Some common examples are (BeH2)n< (AlH3)n, (InH3)n, (SiH4)n, etc.
- Some complex hydrides are also formed. In these hydrides, the hydride ion H– becomes involved in forming a coordinate bond with the central metal atom. B, A1, Ga, etc., of group IIIA form this type of hydride. Some common examples are lithium aluminum hydride (LiAlH4), sodium borohydride (NaBH4), etc. These are extensively used in organic and inorganic chemistry as a versatile reducing agent.