NEET Biology Enzymes
Enzymes. serval definitions given for enzymes are as follows:
- The catalyst in living cells facilitates metabolic reactions.
- The enzymes arc directing and controlling catalysts that determine the particular chemical reaction which makes up the complex metabolic pattern of living organisms.
- A protein with catalytic properties due to its power of specific activation.
- Enzymes are organic catalysts.
Enzymes are proteinic substances that act as biological catalysts with a high degree of specificity produced within the cell having an enormous ability to catalyze all metabolic reactions in a highly effective manner. Enzymes may be simple proteins or conjugated proteins formed of apoenzyme (protein part) and cofactor (prosthetic group or coenzymes or metallic cofactor). Å
Read and Learn More NEET Biology Notes
Enzymes Notes For Neet
Discovery of enzyme
- Edward Buchner (1897) showed that the juice of groundnut and preserved yeast cells ferment sugar.
- The Tenn enzyme was coined by VV. Kuhne.
- J.B. Sumner purified and crystallized urease enzymes from Jack beans. He also suggested that enzymes are proteins.
Properties
- Enzymes function as catalysts, necessitate minimal quantities, exhibit great specificity, and perform optimally at temperatures ranging from 25 to 40°C; low temperatures impede activity, while elevated temperatures lead to denaturation.
- Enzymes necessitate an optimal pH tailored to each individual enzyme. The ratio of enzyme to substrate concentration influences the reaction rate.
- Certain inhibitors impede enzymatic activity. Extracellular enzymes are synthesized in inactive forms known as proenzymes.
- Certain enzymes exhibit minor variations in molecular structure yet execute identical functions; they are referred to as isoenzymes or isozymes.
- For instance, lactic dehydrogenase, which catalyzes the conversion of pyruvate to lactate, possesses five more isomeric forms.
Mechanism of action. Fischer (1894) suggested the Lock and Key hypothesis.
Koshland Induced Fit Theory. Lock and Key Theory (Contemplate theory) has been modified and accepted as induced fit theory. This suggests the existence of two groups i.e. buttressing or supporting and catalytic groups on the active site enzyme.
As the substrate gets associated with the buttressing region, the active site changes configurations so that the catalytic group comes to lie opposite to the area where substrate bonds are to be changed.
NEET Biology Nomenclature And Classification Of Enzymes
According to the International Union of Biochemistry (I.U.B—1961) system of classification.
- Reactions and enzymes catalyzing them are divided into 6 major classes each with 4- 13 subclasses.
- The enzyme name has two parts—the first name is the substrate. The second ending kinase indicates types of reactions.
- The enzyme has a systematic Code No. (E.C.). The first digit denotes the class, the second sub-class, the third sub-sub class and the fourth one is for the particular enzyme name.
Thus E.C.2.7.1.1 denotes class two (transferases)-subclass- 7 sub-subclass 1 fan alcohol function as phosphate acceptor). The fourth digit indicates hexokinase.
Enzymes Notes For Neet
Major Classes of Enzymes
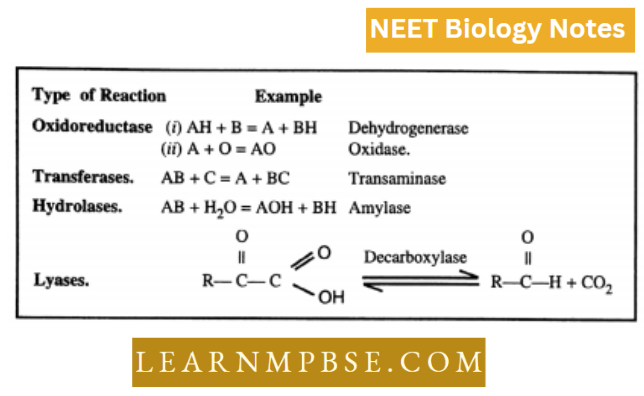
- Oxidoreductases. Formerly known as oxidases and dehydrogenase
- Transferases. Catalyze transfer of one carbon group, aldehyde or ketone, or functional group.
- Hydrolases. These enzymes cause cleavage of a variety of bonds by the addition of water.
- Lyases result in the direct removal of groups from the substrate or the break of the bonds without the addition of water.
- Isomerases. Which catalyze isomeric changes.
- Ligases or Synthetases. Catalyze linking together of different types of bonds.
NEET Biology Some Terms Regarding Enzymes
Isozymes (Isoenzymes):
Enzymes execute identical duties yet exist in several molecular forms within the same tissue or organ.
For instance, lactate dehydrogenase (LDH) comprises five isoenzymes, while alpha-amylase contains sixteen isoenzymes.
- Denaturation: A process or series of actions that alters the structural configuration of the polypeptide chain of a protein molecule from its original state to a more disordered arrangement. The denatured enzyme exhibits no enzymatic activity.
Holoenzyme. The enzyme complex is referred to as a holoenzyme. - Apoenzyme: The protein component of the holoenzyme is thermolabile and colloidal.
- Allosteric locus: A segment of the enzyme where a specific effector or modulator may bind, which can exert either positive or negative effects.
- Allostery (Allosteric inhibition): Modulator-induced alteration of the active site, hence inhibiting substrate binding.
Factors Affecting Enzyme Action
- Effect of enzyme and substrate concentration. Raising the concentration of enzyme increases the rate at which substrate is converted to product, but at high substrate concentration, the enzyme molecules become saturated.
- K1: Disassociation constant of enzyme-inhibitor complex. It applies to competitive inhibitors. Lower K1 is essential for enzyme activity, and higher decreases it.
- Michaelis Constant. (Km) Leonor Michaelis and Mand Menton (1913). It is a constant that indicates the substrate concentration at which the chemical reaction catalyzed by an enzyme attains half of its maximum velocity. The lower the value, the higher the affinity of the enzyme for the substrate.
- Effect of pH. The pH sensitivity of an enzyme depends on how many ionizable R groups are necessary for its activity and its charge. Generally, enzymes function at near-neutral pH.
- Effect of temperature. Temperature above 60°C denatures most proteins or disrupts
their structure. At low temperatures, enzymatic reactions proceed slowly. The optimum
temp is 25- 40 - Some blue-green algae live on the surface of glacial ice and have enzymes adapted to temperatures close to freezing point.
- Some bacteria inhabit hot sprigs of the Yellow Stone Park and their enzymes are adapted to function at temperatures 80-85ºC
Enzyme Classification And Functions Neet
Regulation of Enzyme Action
- Control at the enzyme level. level DNAl so is termed master feedback.
- Control at the gene level. molecule inhibition which controls or negatively the feedback synthesis of proteins and therefore enzymes. Proteins are the end products of gene expressions.
NEET Biology Commercially Important Enzymes
- The biological washing powder enzyme used is Proreases.
- In brewing, amylases found in germinating barley digest starch in the grain to form malt sugar (maltose).
- This sugar is fermented by yeast during brewing
- Amylases used in dishwashing powder remove starch smears from utensils.
- Rennin plays a role in curdling milk thus helping in cheese making.
- In leather making, proteases remove hairs from the hide.
Significant Historical Facts of Enzymes
- Krichoffiis (1815): First indicated the occurrence of enzymes in a living system.
- Louis Pasteur (1860): Fermentation of foodstuffs can be brought about by yeast cells.
- Kuhne (1878): First gave the term ‘Enzymes’.
- Buchner (1897): First prepared a pure extract of zymase enzyme from yeast.
- Sumner (1926): Found enzymes to be proteinaceous, crystallized enzyme urease from Jack beans (Canavalia ensifonnis)
- Northelop (1930): Pure crystals of enzyme pepsin and trypsin from gastric and pancreatic juice.
- Monod et al (1965): structure of Allosteric enzymes. These enzymes do not obey Michaelis Menton’s constant i.e. constant.
- Lock and Key hypothesis was given by Emil Fischer (1894) and modified by Koshland (1971) as induced fit theory.
- Arber Nathans and Smith: discovered, the enzyme Restriction Endonuclease.
- Cecil et al (1981) in ribozyme and Altaman et al (1983) for ribonuclease —P found enzyme activity to be present in RNA. (Non-proteinic enzyme) 03 Hansen—Isolated renin Indicate Nobel Laureate.
Enzyme Classification And Functions Neet
- Enzymes are biological catalysts.
- All enzymes have a specific three-dimensional structure, a part of which is known as an active site.
- An enzyme may have more than one active site.
- Formation of carbonic acid (H2, CO3) from CO, and H,0 in the presence of carbonic anhydrase is 10 million times faster than the non-catalysed reaction.
- Each enzyme can catalyze the change of either a specific substrate or a specific group of a substrate.
- Each enzyme shows its highest activity at a specific pH.
- Cyanide kills an animal by inhibiting cytochrome oxidase, a mitochondrial enzyme essential for cellular
- Respiration. It is an example of non-competitive inhibition of an enzyme.
- The decline in enzyme activity by the allosteric effect of the product is called feedback inhibition Example allosteric inhibition of hexokinase by glucose-6-phosphate.
- Competitive inhibitors are always reversible.
- Non-competitive inhibitors may be reversible or permanent.
c& Non-reversible inhibitors are always non-competitive. - According to the Michaelis-Menten equation Km is equal to substrate concentration at which half of maximum velocity is achieved. Michaelis and Menten proposed a
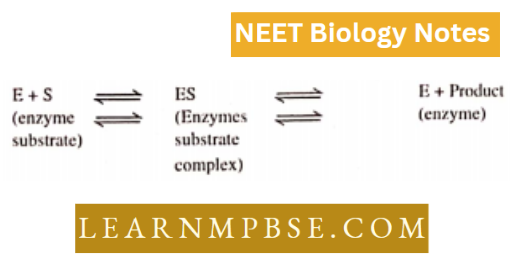
- Hypothesis For Enzyme Action According to the enzyme molecule combines with a substrate complex which further dissociates to form a product and enzyme back
- \(\mathrm{E}+\mathrm{S} \underset{K_2}{\kappa_1} \mathrm{E}-\mathrm{S} \rightarrow{K_3} \mathrm{E}+\mathrm{P} ;\)
- Km (Michaelis-method constant)= \(=\frac{K_3+K_2}{K_1}\)
- They gave the following equation:
- \(V_o=\frac{V_{\max }(S)}{(S)+K_m}\)
- It is the statement of the quantitative relationship between the initial velocity Vo, the maximum velocity Vmax, and the initial substrate concentrations: all related through Michaelis Menten constant Km
- H- free enzyme
- S = substrate
- HS = enzyme-substrate complex
- I = product
- K1= (he rate constant for ES formation
- K2 = the rate constant for the dissociation of ES into E and S
- K1 is the rate constant for the dissociation of the ES complex into ES and P.
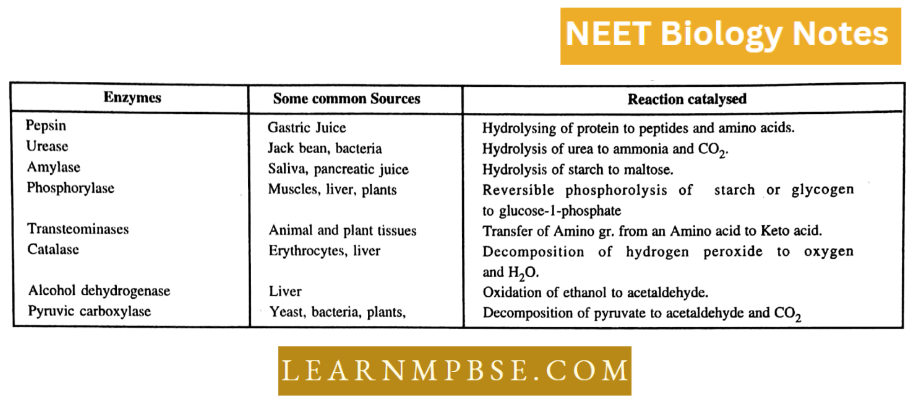
Enzyme Inhibition And Regulation Neet Biology
Quanta to memory
- Kuhne (1878) coined the term enzyme.
- Buchner ( 1897) isolated enzymes for the first time. All components of the cell including the cell wall and cell membrane have enzymes.
- Mitochondria contain a maximum number of enzymes in the cell. (70%)
- The smallest enzyme is peroxidase and the largest is catalase found in peroxisomes.
- Enzyme activity increases from 0°C to optimum temperature and doubles with every 10° rise in temperature. This is called the temperature coefficient (Q10)
- Chemozymes are chemically synthesized enzymes.
- The highest turnover number is of the enzyme carbonic anhydrase. (36 million) with Zn++ as. activator.
- The lowest turnover number is of enzyme lysozymes.
- Mental Activators
- Na for AT Pase
Fe „ Catalase, aconitase, cytochrome oxidase
Zn „ Carbonic anhydrase
Mg „ Hexokinase
Mo „ Nitrate reductase - Enzyme urease isolated from Jack bean Canavalia was crystallized by Sumner in 1926, who proved the protein nature of enzymes.
- Enzymes show 3-D structure.
- Enzymes work in milliseconds and the rate of enzyme of the substrate is as high as 1: 10,00,000.
- Now RNA with catalytic and synthetic functions has been found in a protozoan Tetrahymena thermophila.
- The smaller the Km, the greater the substrate affinity.
Turn over Numbers
- Metal Activators
- Carbonic anhydrase = 3.6 x 106
- Acetyl cholinesestrase = 1.5 x 106
- Urease = 1.0 x l06
- Amylase = 10 x 10s
- Lactic acid dehydrogenase = 6.0 x 104
- Chymotrypsin = 6.0 x 103
- Lysozyme = 3.0x 101
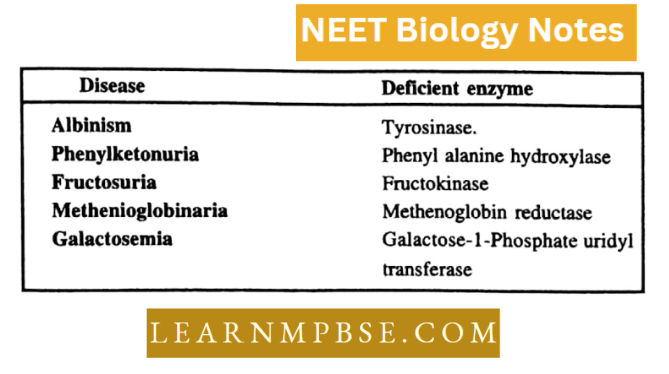
Enzymes Identified with Hereditary Disease