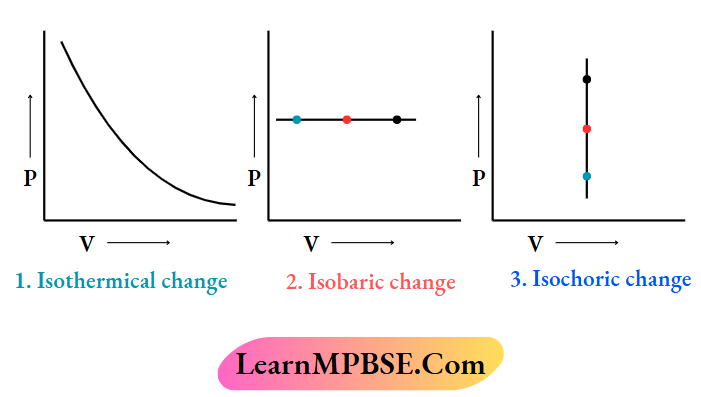
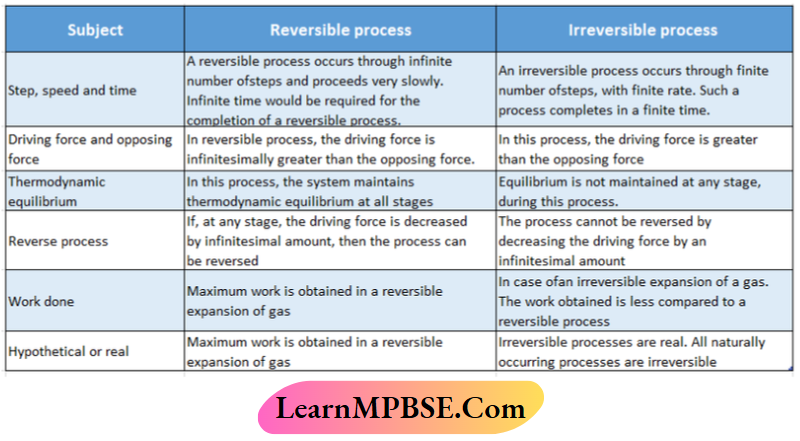
MPBSE Class 11 Chemistry Chapter 7 Equilibrium Question And Answers
Question 1. A liquid is in equilibrium with its vapor at its boiling point. On average which property of the molecules is equal in two phases?
Answer:
At the boiling point of a liquid in equilibrium with its vapor, the average kinetic energy of the molecules in the two phases is equal.
Question 2. According to Le Chatelier’s principle, what is the effect of adding heat to a solid and liquid in equilibrium?
Answer:
In the equilibrium system solid-liquid, the forward process is endothermic. Therefore, if temperature is increased at equilibrium, then, according to Le Chatelier’s principle, equilibrium will shift to the right, thereby increasing the amount of liquid.
Question 3. Mention two ways by which the equilibrium of the L-given reaction can be shifted to the right.
Answer:
According to Le Chatelier’s principle, the equilibrium of the above reaction can be shifted to the right by the addition of excess reactants [i.e., CH3COOH(l) or C2H5OH(l) ] or by the removal of the products [i.e., CH3COOC2H5(l) or H2O(l)] from the reaction system at a given temperature, keeping the volume of the reaction system constant.
Read and Learn More Class 11 Chemistry
Question 4. When steam is passed over a red-hot iron, H2 gas is produced. In this reaction, the yield of H2(g) is found to increase when the partial pressure of steam is increased. Explain.
Answer:
Reaction:
⇒ \( \mathrm{Fe}(s)+4 \mathrm{H}_{-} \mathrm{O}(\rho) \rightleftharpoons \mathrm{Fe}_{-} \mathrm{O} \cdot(\mathrm{s})+4 \mathrm{H}_{-}(g)\)
A steam is one of the reactants in the above reaction, increasing its partial pressure at equilibrium will shift the equilibrium position to the right. As a result, the yield of the product i.e., H2(g) will increase.
Class 11 Chemistry Chapter 7 Equilibrium
Question 5. Why does not the equilibrium constant expression for a reaction involving pure solids or liquids contain the concentration terms of the solids or liquids?
Answer:
For a pure solid or liquid, molar concentration is directly proportional to density. Given that density remains constant at a specific temperature, the molar concentration of a pure solid or liquid at that temperature is a constant value, typically regarded as unity (1). Consequently, the equilibrium constant expression for a process involving pure solids or liquids excludes concentration terms for these phases.

Question 6. At constant temperature, the following reaction is at equilibrium in a closed container: \(\mathrm{C}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightleftharpoons \mathrm{CO}(\mathrm{g})+\mathrm{H}_2(\mathrm{~g})\) At constant temperature, if the amount of the solid carbon is reduced to half at equilibrium, then what will be the change in the concentration of CO(g)?
Answer:
At a particular temperature, the concentration of any pure solid is independent of its amount. Thus, keeping the temperature constant, if the amount of solid carbon is reduced to half at equilibrium, then its concentration will remain unchanged. So, the concentration of CO(g) will also remain unaffected.
Mpbse Solutions For Class 11 Chemistry Chapter 7 Equilibrium
Question 7. The values of the equilibrium constant (if) of a reaction at 25°C & 50°C are 2 ×10-4 & 2 ×10-2, respectively. Is the reaction exothermic or endothermic?
Answer:
With the increase in temperature, the value equilibrium constant (K) increases for an endothermic reaction, while it decreases for an exothermic reaction. For the reaction, K(50°C) > K(25°C), indicating it is an exothermic reaction.
Question 8. For a gaseous reaction, Kp > Kc. What will be the effect on equilibrium if pressure is increased at a constant temperature? Will it affect the yields of the products?
Answer:
According to the relation Kp = Kc(RT)-Δn, if Kp> Kc, then Δn > 0. The positive value of An implies that the reaction occurs with an increase in volume in the forward direction. For such a reaction, if pressure is increased at equilibrium, then according to Le Chatelier’s principle the equilibrium will shift to the left and thus the yield ofthe product will decrease.
Question 9. In the case of the thermal decomposition of H2(g) to H(g), which conditions of pressure and temperature will be favorable for an increase in the yield of H(g)?
Answer:
Since the formation of H(g) from H2(g) [H2(g) 2H(g)] occurs through decomposition, it is an endothermic reaction. Because 2 moles of H(g) are formed from 1 mole of H2(g), the reaction is associated with a volume increase. So, according to Le Chateliehs principle, the yield of H (g) will increase if the reaction is carried out at a high temperature and low pressure.
Chemical Equilibrium Class 11 Ncert Solutions
Question 10. In the case of the reaction A2(g) + 4B2(g), 2AB4(g), the change in enthalpy (ΔH) is negative. Mention the conditions of pressure and temperature at which the yield of the product, AB4(g) will decrease.
Answer:
Since ΔH < 0, it is an endothermic reaction. The volume of the reaction system decreases in the forward direction [1 molecule of A2(g) combines with 4 molecules of B2(g) to form 2 molecules of AB4(g)]. Thus; according to Le Chatelier’s principle, under the conditions of high temperature and low pressure, the yield of AB4(g) will decrease.
Question 11. How will the equilibrium of the reaction, H2(g) + I2(g) ⇌ 2HI(g) be affected if the volume of the reaction system at equilibrium is doubled, keeping the temperature constant?
Answer:
Doubling the volume of the reaction system at equilibrium will reduce the total pressure of the system by half. But for the reaction Δn = 0. So, according to Le Chatelier’s principle, the equilibrium ofthe reaction will not be affected by a change in pressure.
Question 12. In the reaction, I2+I–→I3–, which one acts as a Lewis base?
Answer:
In the reaction between and I2, the I– ion donates an electron pair to the I2 molecule, resulting in the formation of the I3– ion [I2+I–→I3– ]Therefore, the I– ion acts as a Lewis base in this reaction.
Question 13. The pKa values of the three weak acids HA, HB, and HC are 4.74, 3.75, and 4.20, respectively. Arrange them in order their of increasing acid strengths.
Answer:
As pKa = -log10 Ksp, the smaller the value of Ka the larger the value of pKa. So, an acid with a larger pKa will have a smaller Ka. As, pKa(HB) < pKa(HC) < pKa(HA) , pKa(HB) > Kfa(HC) > Ka(HA). At a certain temperature, a higher value of Ka for an acid indicates a higher strength of the acid. Therefore, the increasing order of acid strengths of the given acids will be — HA < HC < HB.
Question 14. X and Y are two aqueous solutions of added HA with concentrations of 0.1 M & 0.01M, respectively. In which solution will the degree of ionization of U A be higher
Answer:
According to Ostwald’s dilution law, the degree of ionization of a weak electrolyte increases with the increase in dilution of its aqueous solution. Since, the concentration of solution Y is less than that of X, the degree of ionization of HA will be higher in solution Y.
Chemical Equilibrium Class 11 Ncert Solutions
Question 15. Which one of the following two acids will have a higher concentration of H3O+ ions in their 0.1(M) aqueous solutions HCl and CH3COOH?
Answer:
HCl is a strong acid, while CH6COOH is a weak acid. Thus, HCl ionizes almost completely in aqueous solution, whereas CH3COOH undergoes partial ionization. As a result, the concentration of H30+ ions in 0.1(M) HCl solution is higher than that in 0.1(M) CH3COOH solution.
Question 16. Show that [OH-]>\(\sqrt{K_w}\) in an alkaline solution.
Answer:
We know, [H3O+] × [OH– ] = Kw. In pure water, [H3O+] = [OH–].
This gives \(\left[\mathrm{H}_3 \mathrm{O}^{+}\right]=\left[\mathrm{OH}^{-}\right]=\sqrt{K_w}\)
In an alkaline solution, the concentration of OH– ions is higher than that in pure water.
Therefore, in an alkaline solution, \(\left[\mathrm{OH}^{-}\right]>\sqrt{K_w} \text {. }\).
Question 17. Will the concentration of HgO+ ions in pure water at 0°C be more than or less than that at 4°C?
Answer:
Ionization of water is an endothermic t process: [2H2O(1) H3O+(aq) + OH–(aq)]. Hence, with, a temperature rise, the ionic product of water (Kw) increases. Therefore, KM,(4°C) In pure water, [H3O+] = Jÿw- Since,(4°C) > Kw(0°C) , the concentration of H2O+ ions in pure water at 4 °C will be higher than that at 0°C.
Question 18. At a certain temperature, what is the value for the die sum of pH and pOH for an aqueous solution? What will be its value at 25°C?
Answer:
In case of any aqueous solution at a certain temperature pH+POH=pkw. At 25C, Pkw= 14. Hence at 25C PH+POH=14.
Mpbse Class 11 Chemistry Notes Pdf
Question 19. An acid bottle labeled pH – 5 Is this acid a weak acid?
Answer:
The acid may be a weak add or a very dilute strong acid. The pH of an added solution depends upon the die concentration of H3O+ Ions in the solution. So, from the value of pH, it Is not possible to predict whether the acid IN is weak or strong.
Question 20. A, B, and C are three buffer solutions, each of which is composed of a weak acid and its salt. For increasing the pH by 0.02 units, it is found that 1.0, 1.4, and 1.2 millimol of NaOH are required for A, B, and C, respectively. Arrange the solutions in the increasing order of their buffer capacities.
Answer:
The higher the buffer capacity of a buffer solution, the greater the amount of a strong acid or a strong base to be required for increasing the pH of the buffer. It is given that increasing the pH of the buffer by the same amount requires a minimum amount of NaOH for buffer A and a maximum amount of NaOH for buffer B. Therefore, the increasing order of buffer capacity of the given buffers is A < C < B.
Question 21. Of the two bottles, one contains an HCl solution and the other a buffer solution. Each of the bottles b labelled as pH = 5. How can you identify the solutions?
Answer:
Upon measuring the pH of the solutions following the addition of equal drops of NaOH, a significant increase in pH will be observed for one solution, whilst the pH of the second solution remains relatively unchanged. A buffer solution maintains a relatively constant pH, whereas a solution of HCl results in an increase in pH.
Question 22. At a certain temperature, the Ksp of AgCl in water is 1.8 × 10-10. What will be its Ksp is a 0.1M solution of AgNO3 at some temperature.
Answer:
At a certain temperature, the solubility of AgCl decreases in the presence of a common ion (Ag+), but the solubility product of AgCl remains the same. Therefore, Ksp for AgCl in 0.1(M) aqueous solution of AgNO3 will be the same as that in water.
Question 23. You are supplied with HCOOH (pKa =3.74), CH3COOH (pKa = 4.74), and NaOH solutions. To prepare a buffer solution of pH =3.8, which acid will you select? Give reason
Answer:
The buffer capacity of a buffer solution consisting of a weak acid and its salt becomes maximum when the pH ofthe buffer solution is equal to the pKa of the weak acid. Among the given acids, the pKa of HCOOH is very close to the desired pH of the buffer solution. Hence, one should use HCOOH for preparing the buffer.
Question 24. pH of a buffer solution composed of NH3 and NH4Cl is 9.26. Will there be any change in pH if 100 mL of distilled water is added to 100 mL of this buffer solution?
Answer:
For the buffer solution made up of NH3 and NH4Cl, the pH of the solutions is given by
⇒ \(p H=14-p K_b-\log \frac{\left[\mathrm{NH}_4 \mathrm{Cl}\right]}{\left[\mathrm{NH}_3\right]}\)
If 100 mL of distilled water is added to 100 mL of this buffer solution, no change occurs in the ratio of [NH4Cl] to [NH3] and the pH ofthe solution remains the same.
Mpbse Class 11 Chemistry Notes Pdf
Question 25. Which of the given salts will undergo cationic or anionic or both cationic and anionic hydrolysis? NH4F, NaCN, AICl3, Na2CO3, NH4Cl
Answer:
Both NH4Cl and AlCl3 are the salts of strong acids and weak bases. In aqueous solution of such salts, cationic hydrolysis takes place. NaCN and Na2CO3 are the salts of weak acids and strong bases. In an aqueous solution of such salts, anionic hydrolysis takes place. NH4F is a salt of weak acid and weak base. In aqueous solution of such salt, both cationic and anionic hydrolysis take place.
Question 26. The values of pure water at 0°C and 25°C are x and y respectively. Is it greater than or less than y?
Answer:
For pure water \(p H=\frac{1}{2} p K_w\) Rise in temperature increases the value of Kw.
So, Kw(0°C) < Kw(25°C) & hence pKw(0°C) > pKw(100°C) as pKw = -log10. As in the case of pure water,
⇒ \(p H=\frac{1}{2} p K_w\) pH of pure at 0 °C will be greater than that at 25 °C. Hence, x > y.
Question 27. pKw = 12.26 at 100°C. What is the range of pH -scale at this temperature? What will be the pH of a neutral solution at this temperature?
Answer:
At 100 °C, pKw = 12.26. So, at this temperature, the pH scale ranges from 0 to 12.26. At this temperature,
⇒ \(\left[\mathrm{H}_3 \mathrm{O}^{+}\right]=\sqrt{K_w}\)
In a neutral aqueous solution.
Therefore, pH of this solution \(=\frac{1}{2} p K_w=\frac{1}{2} \times 12.26=6.13\)
Question 28. Why does the concentration of OH“ ions in pure water increase with temperature rise? Does this increase make pure water alkaline? Explain.
Answer:
In pure water, [OH–] = JKw. Kw increases with temperature, and so does [OH–]. This increase in [OH–] does not however mean that pure water becomes alkaline at a higher temperature as pure water always contains an equal number of H3O+ and OH– ions at any temperature.
Mpbse Chemistry Chapter 7 Important Questions
Question 29. All Lewis bases are fact bases—explain. Each of HCO2 and HPO– can act both as Bronsted acid and hose—why? Write the formula of conjugate base and conjugate acid in each case.
Answer:
A chemical capable of accepting a proton is termed a Bronsted base, whereas a substance that can give a pair of electrons is referred to as a Lewis base. The NH3 molecule is capable of accepting a proton. Thus, it is a Brønsted base. The NH3 molecule provides a pair of electrons to establish a coordination bond with a proton. Thus, it also functions as a Lewis base. Consequently, it may be deduced that a Lewis base qualifies as a Bronsted base.
Question 30. Each of HCO3– and HPO– can act both as Bronsted acid and hose—why? Write the formula conjugate base and conjugate acid in each case.
Answer:
According to the Bronsted-Lowry concept, an acid is a proton donor and a base is a proton acceptor. Since the given species are capable of accepting and donating a proton, they can act as an acid as well as a base.
Question 31. Of the two solutions of acetic acid with concentrations 0.1(N) and 0.01(N), in which one does acetic acid have a higher degree of dissociation?
Answer:
Acetic acid is a weak acid. Such an acid undergoes partial ionization in water. The degree of ionization of a weak acid in its solution depends upon the concentration of the solution. The higher the concentration of a solution of a weak acid, the smaller the degree of ionization of the acid in that solution. So, the degree of ionization of acetic acid will be higher in 0.01(N) solution.
Question 32. At a certain temperature, the ionization constants of three weak acids HA, HB, and HC are 4.0 × 10-5 5.2 × 10-4, and 8.6 × 10-3, respectively. If the molar concentrations of their solutions are the same, then arrange them in order of their increasing strength.
Answer:
The larger the ionization constant (Ka) of an acid, the greater the extent to which it undergoes ionization in its aqueous solution, and hence the higher the concentration of H30+ ions produced by it in the solution. Alternatively, the larger the value of Ka of an acid, the greater its strength.
The increasing order ofthe given acids concerning their Ka: HA < HB < HC. Consequently, the order of the given acids in terms of increasing strength in their aqueous solutions will be HA < HB < HC.
Mpbse Chemistry Chapter 7 Important Questions
Question 33. At 25°C, the ionization constant (Ka) of weak acid HA is 10-6. What will the value of the ionization constant (Kb) of its conjugate base (A-) be at that temperature?
Answer:
We know \(K_a \times K_b=10^{-14} \text { [at } 25^{\circ} \mathrm{C} \text { ]. } K_a \text { of } \mathrm{HA}=10^{-6} \text {. }\)
Therefore \(K_b \text { of } \mathrm{A}^{-}=\frac{10^{-14}}{K_a}=\frac{10^{-14}}{10^{-6}}=10^{-8} .\)
Question 34. Give an example of a salt solution whose pH is independent of salt concentration.
Answer:
In the case of a solution of a salt formed from a weak acid and a weak base, the pH of the solution does not depend upon the concentration of the salt. One such salt is CH3COONH4.
Question 35. For what kind of solids, is solid vapor equilibrium achieved easily?
Answer:
Solid substances that can be easily converted to vapors on heating under normal pressure (i.e., sublimable substances).
For example: Solid CO2, camphor, ammonium chloride, naphthalene, etc.
Question 36. At a fixed temperature, a liquid is in equilibrium with its vapors in a closed vessel. Which measurable tint quantity for the liquid gets fixed at equilibrium?
Answer:
When a liquid remains in equilibrium with its vapor in a closed vessel at a particular temperature, the vapor pressure of the liquid is found to acquire a fixed value.
Mpbse Chemistry Chapter 7 Important Questions
Question 37. At 0°C and 1 atm pressure, why is the equilibrium established between water and ice regarded as dynamic?
Answer:
At 0°C and under 1 atm pressure, ‘water ice’ equilibrium is said to be dynamic since at equilibrium the rate of melting of ice is equal to the rate of freezing of water.
Question 38. Give two examples of chemical reactions for each of the following cases:
- Kp > Kc
- Kp<Kc
- Kp = Kc
Answer:
We know, Kp = Kc(RT)Δn
Kp will be greater than Kc if Δn > 0. Reactions for
Question 39. The following reaction is carried out in a closed vessel at a fixed temperature: A(g) 2B(g). The concentrations of A(g) and B (g) in the course ofthe reaction are as follows When does the reaction attain equilibrium? What are the equilibrium concentrations of A and B?
Answer:
From the time of 60 minutes, the concentrations of reactant and product are found to remain the same with time. Therefore, the reaction has arrived at an equilibrium state at 60 minutes.
At the state of equilibrium, [A] = 0.58 mol- L-1 and [B] = 0.84 mol- L-1
Question 40. Here are a few salts. Whose aqueous solution(s) at 25°C has (have) a pH greater than 7, less than 7, or equal to 7? (NH4)2SO4, CH3COONH4, K2CO3, NaNO3
Answer:
pH < 7 : (NH4)2SO4
pH> 7 : K2CO23
pH = 7 : CH3COONH4, NaNO3